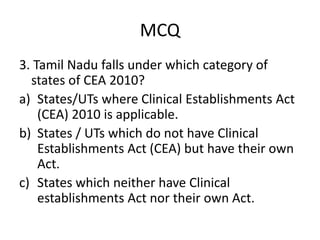
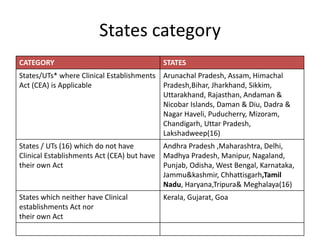
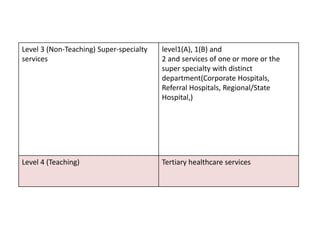
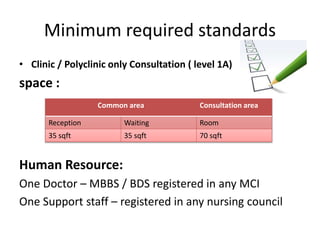
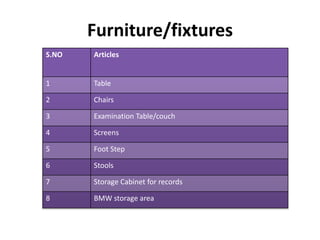
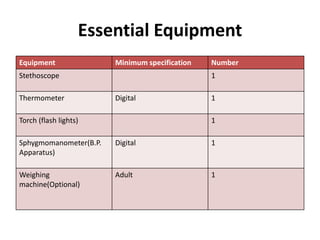
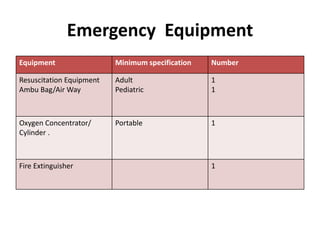
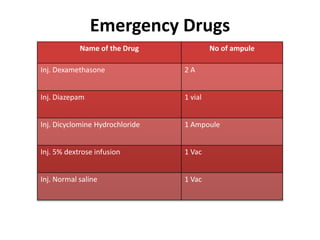
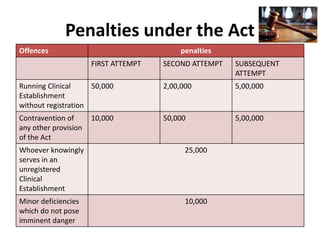
The document defines clinical establishments and classifies them into different levels. It discusses the Clinical Establishments Act passed in 2010 to regulate healthcare facilities in India. The act established authorities at the national, state, and district levels to register clinics and enforce minimum standards. It classifies Tamil Nadu as a state that has its own clinical establishments act. The document also outlines requirements for registering a clinic in Tamil Nadu, including online registration procedures, minimum space, staffing, equipment, and drug requirements.