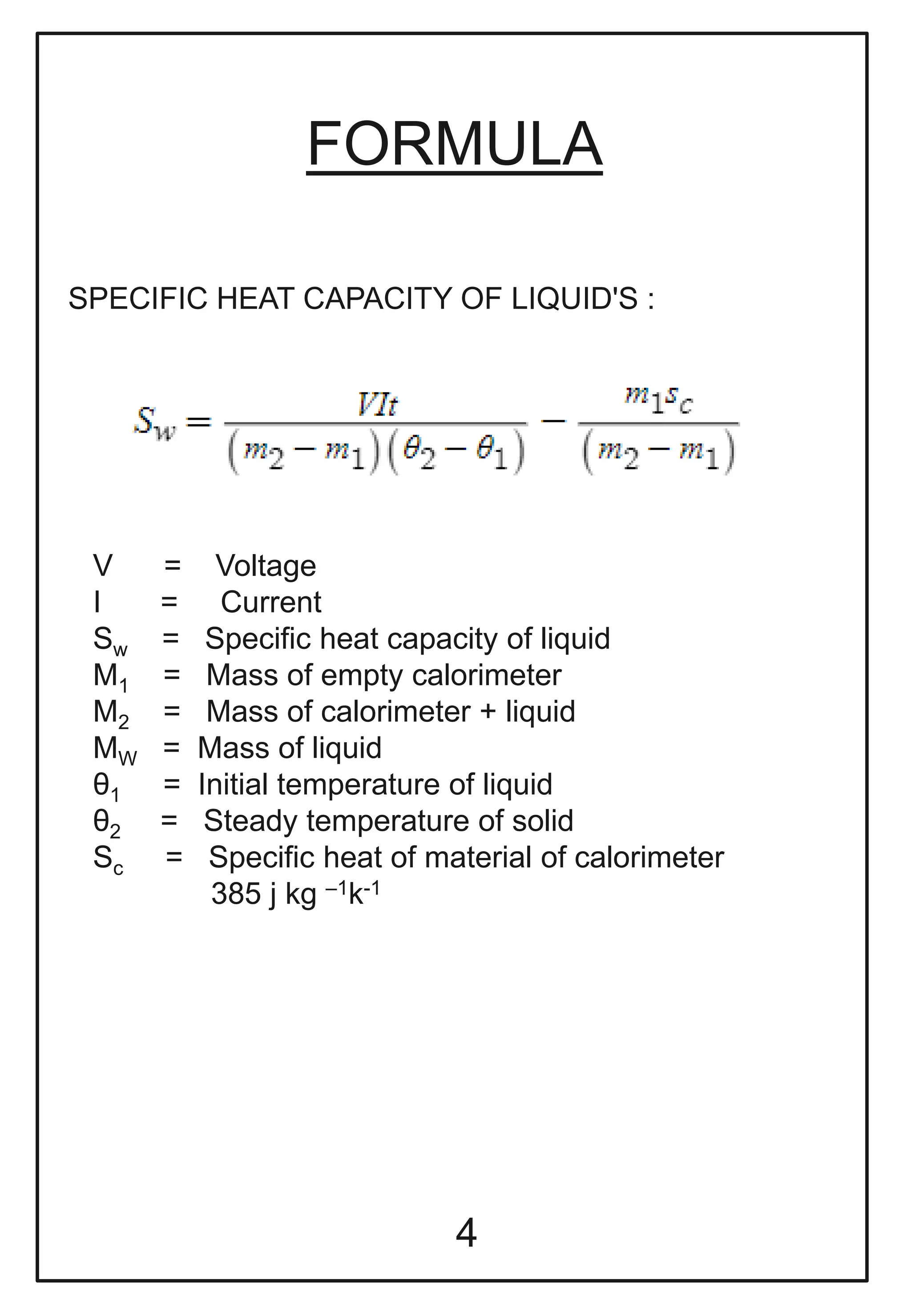
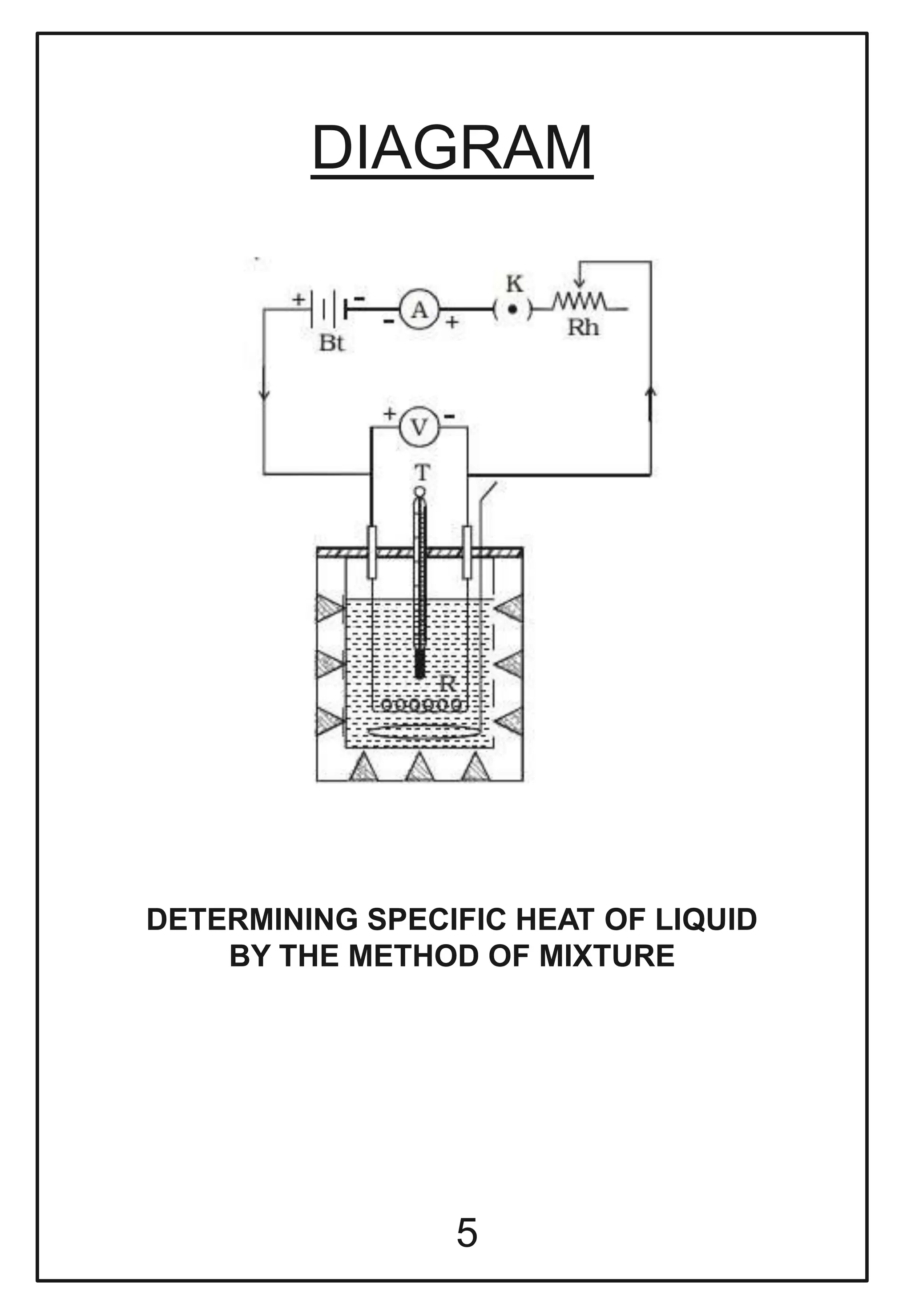
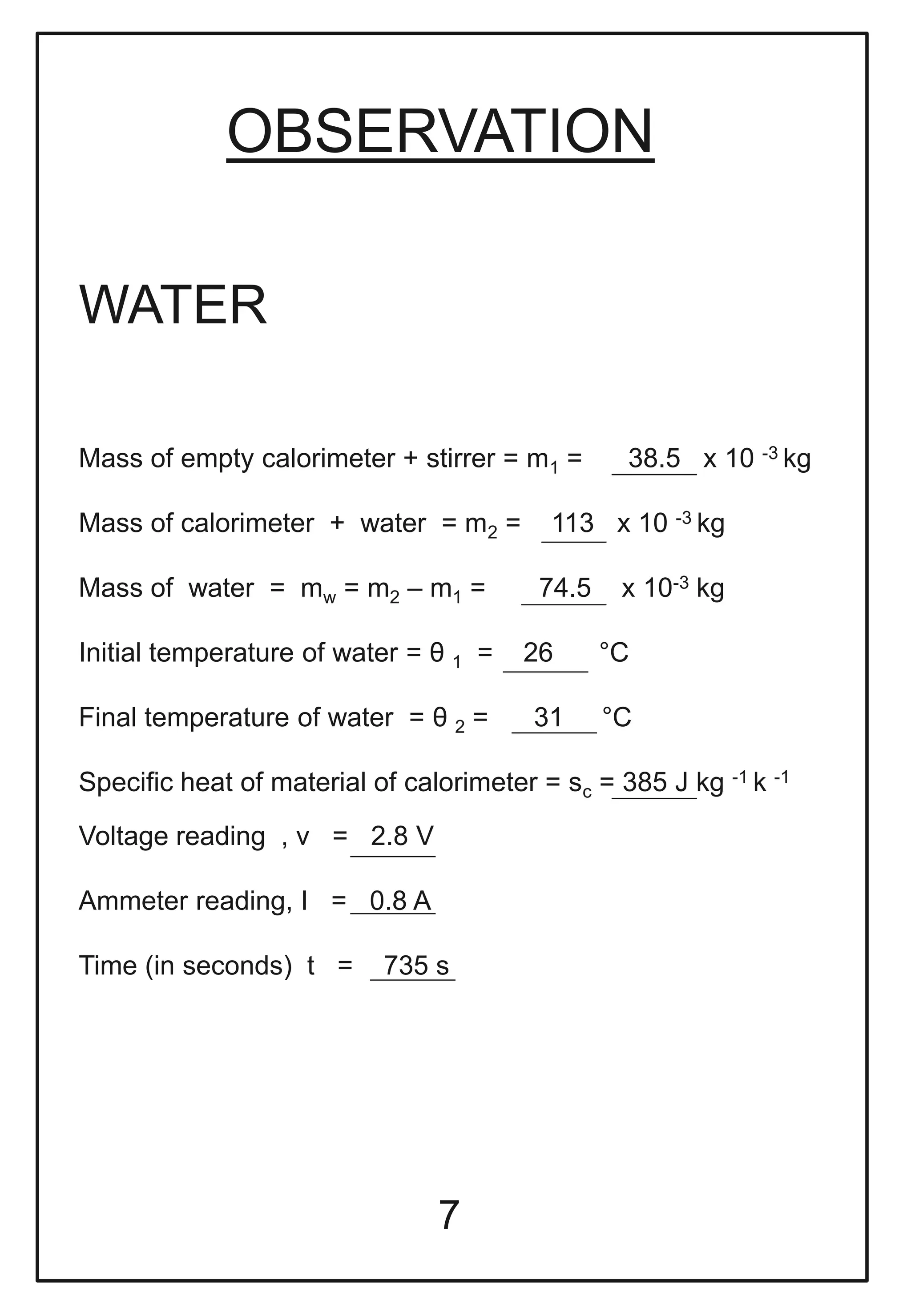
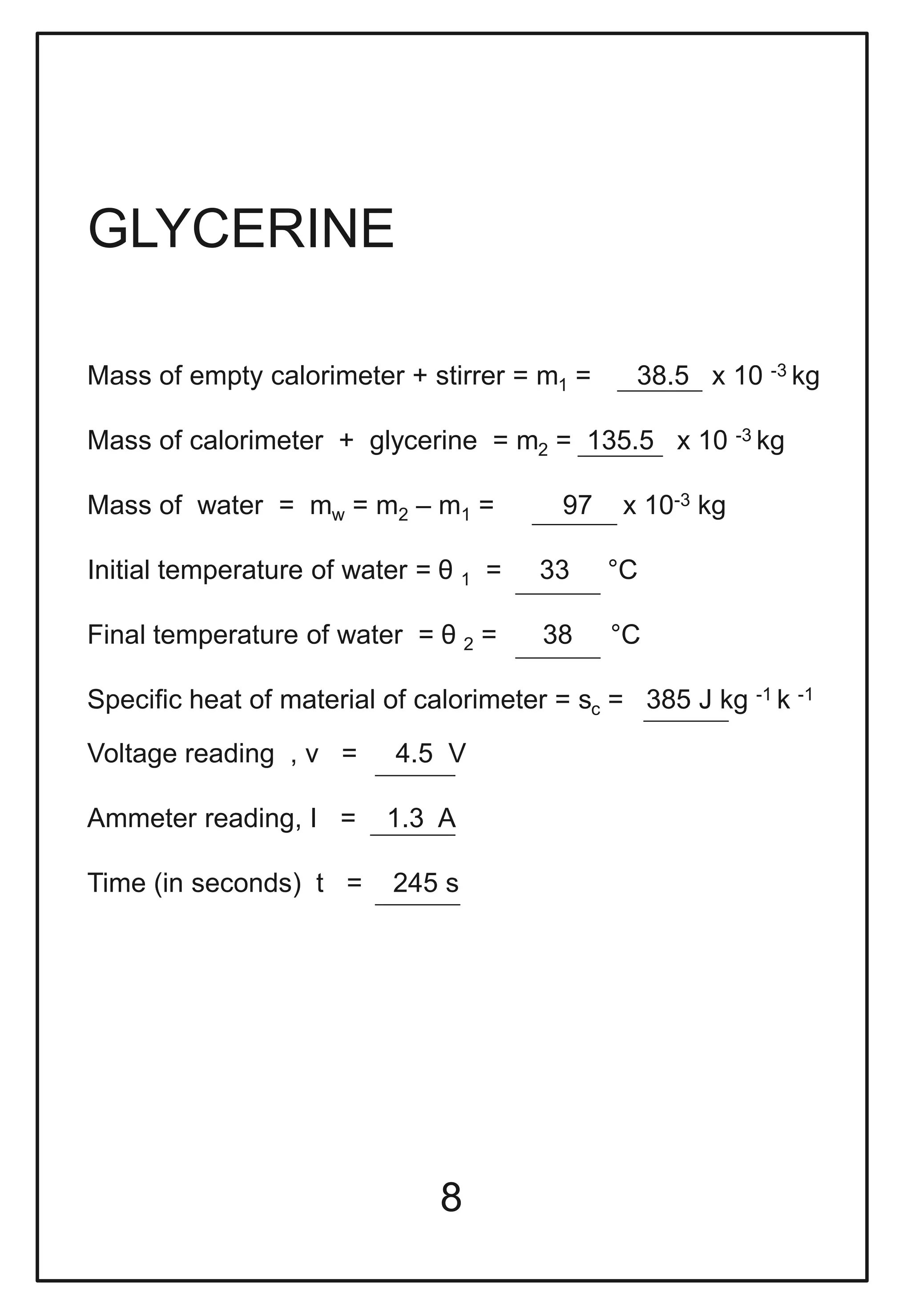
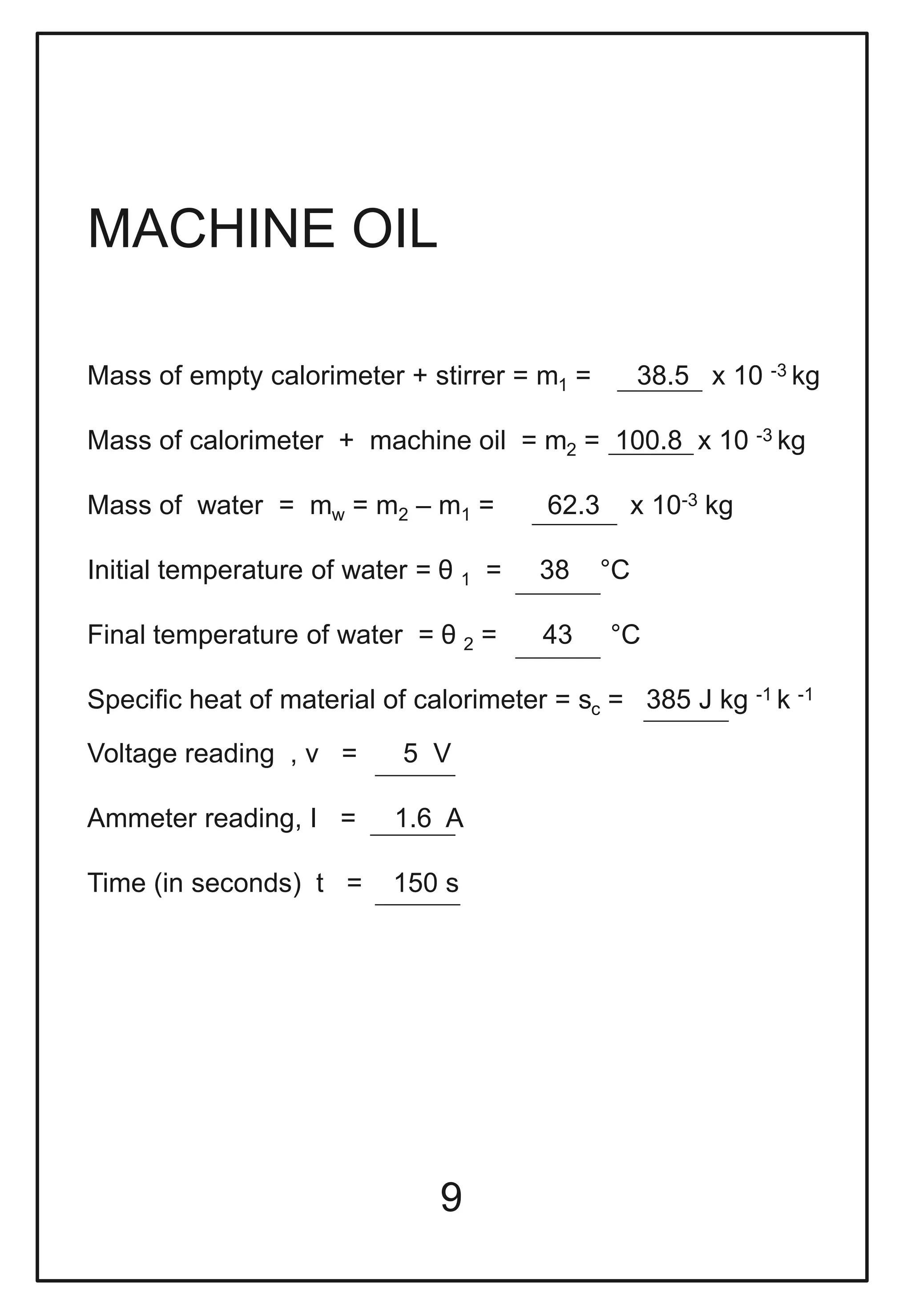
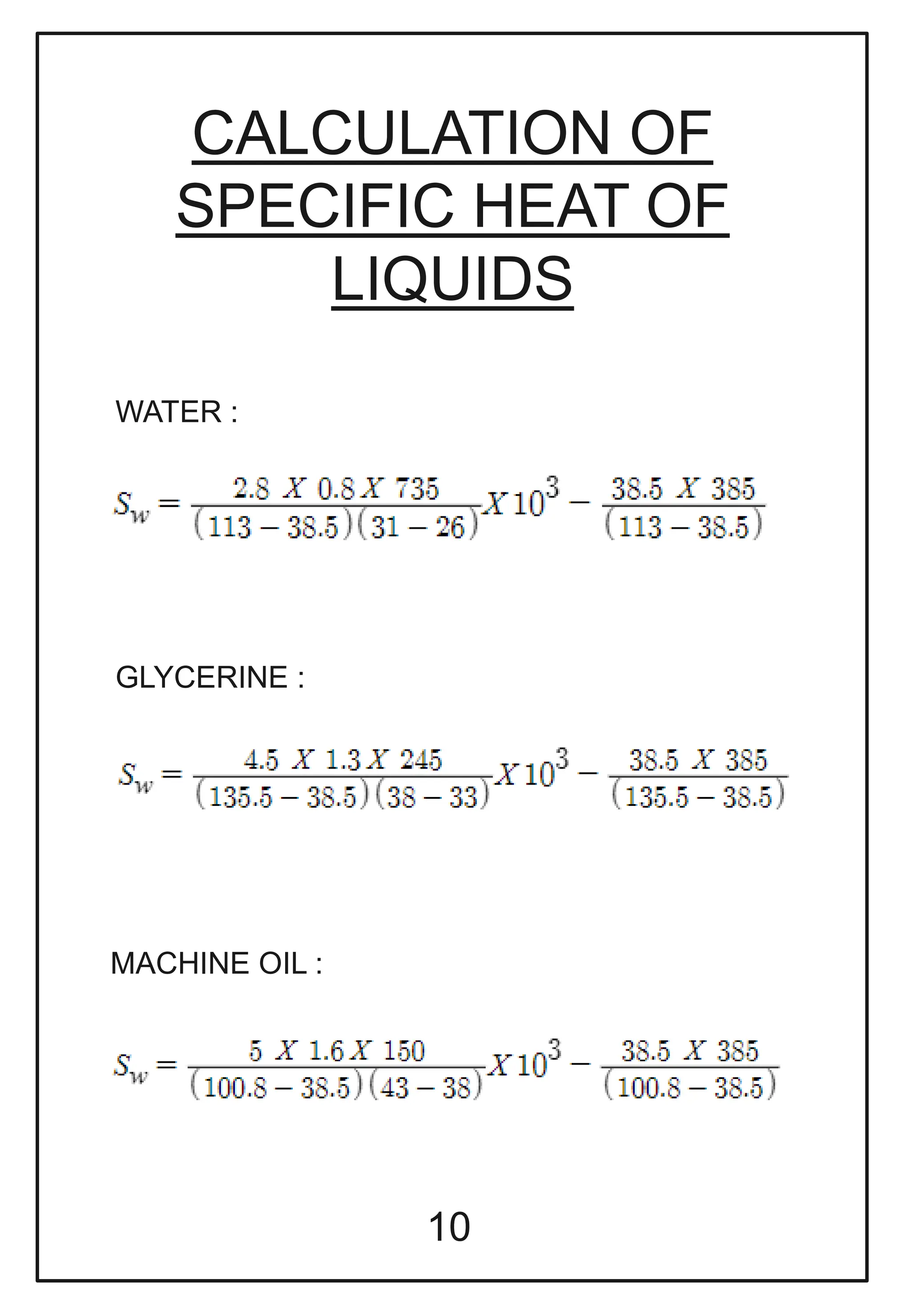
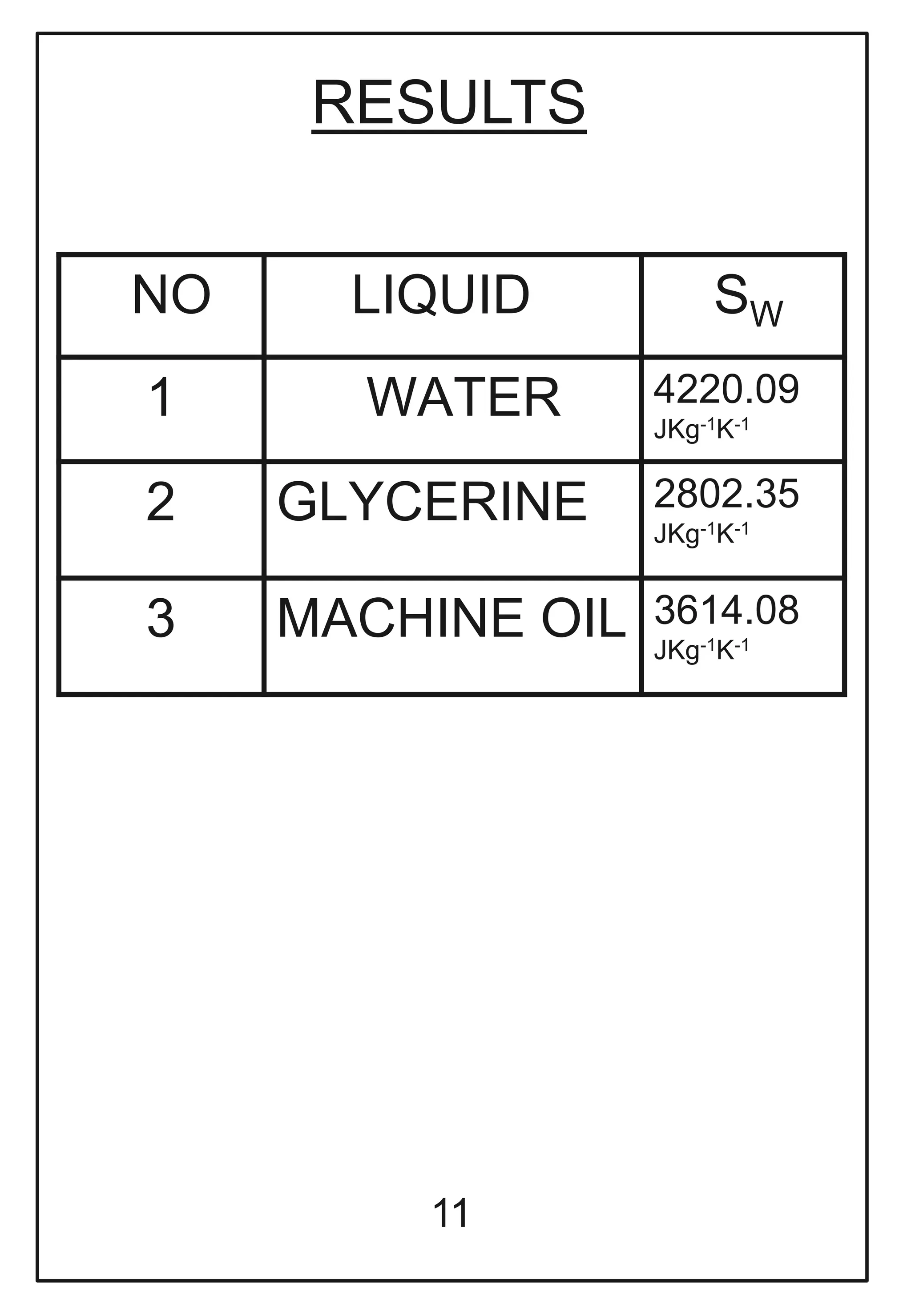
This document describes a physics investigatory project to determine the specific heat of different liquids using Joule's calorimeter. The student measures the mass, initial and final temperatures, voltage, current and time for water, glycerine and machine oil. Calculations are shown to find the specific heat of each liquid. The results show the specific heat of water is 4220.09 J/kg°C, glycerine is 2802.35 J/kg°C, and machine oil is 3614.08 J/kg°C.