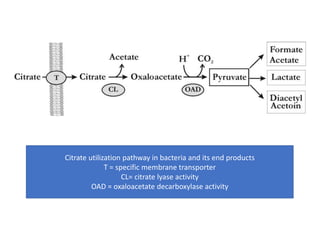
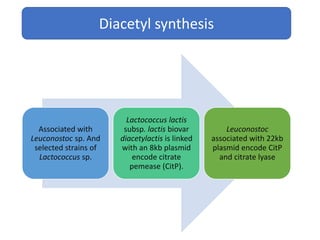
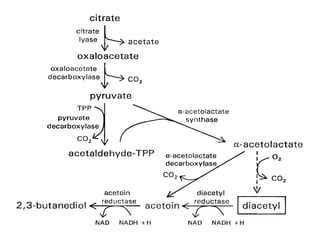
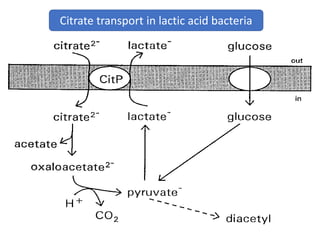
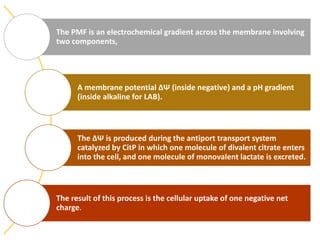
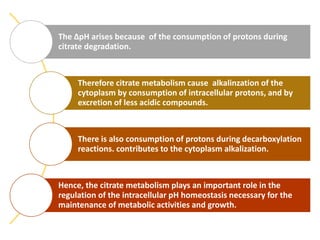
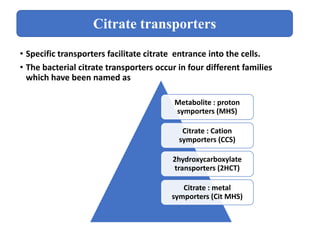
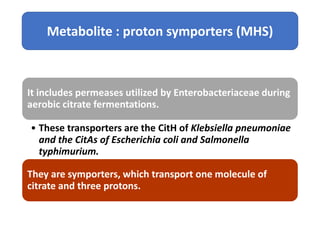
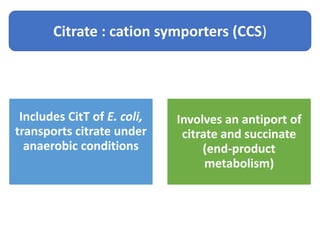
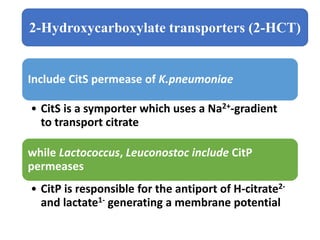
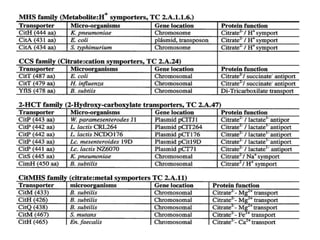
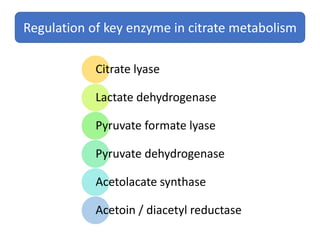
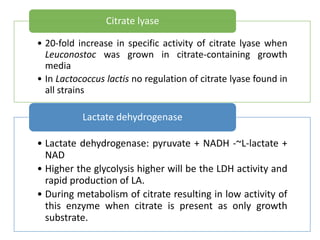
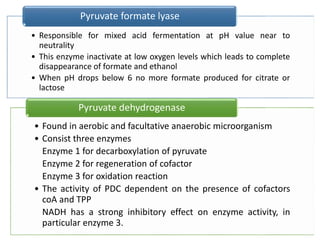
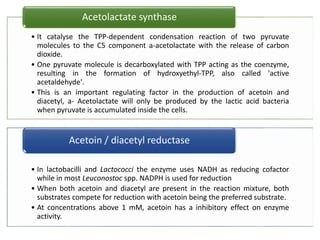
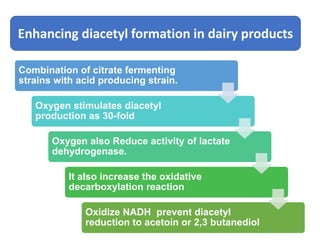
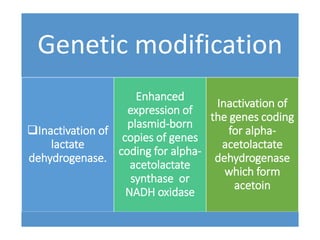
Citrate metabolism produces various end products including acetate, diacetyl, acetoin, and butanediol. Citrate is transported into bacteria via citrate permeases and metabolized through pathways involving citrate lyase and other enzymes. This metabolism generates a proton motive force through citrate/lactate antiport and consumption of protons during decarboxylation reactions. Regulating enzymes in the pathway and combining citrate fermenters with acid producers can enhance diacetyl formation, an important flavor component in dairy products. Genetic modifications can also be used to modify citrate metabolism and increase desirable end products.