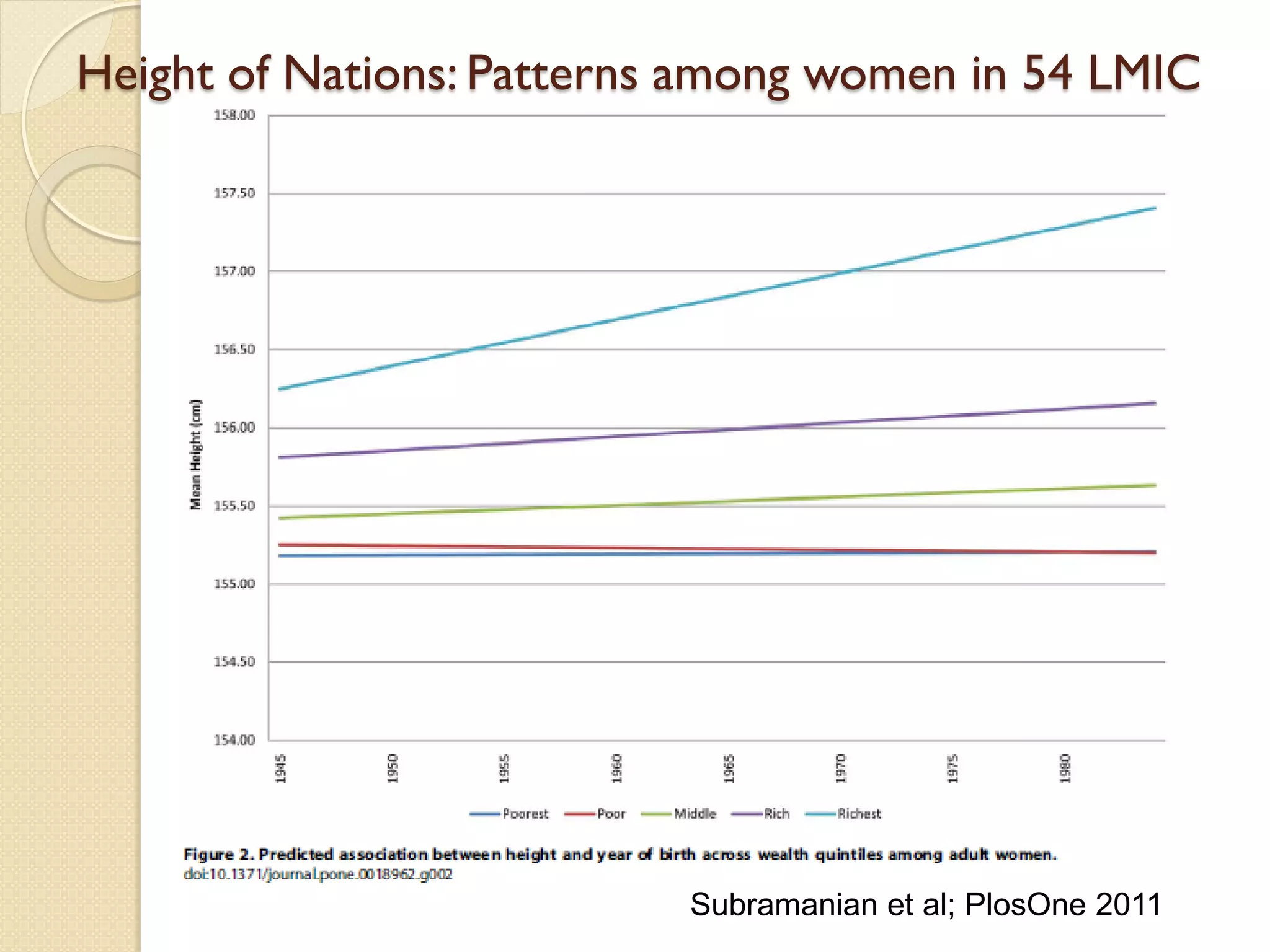
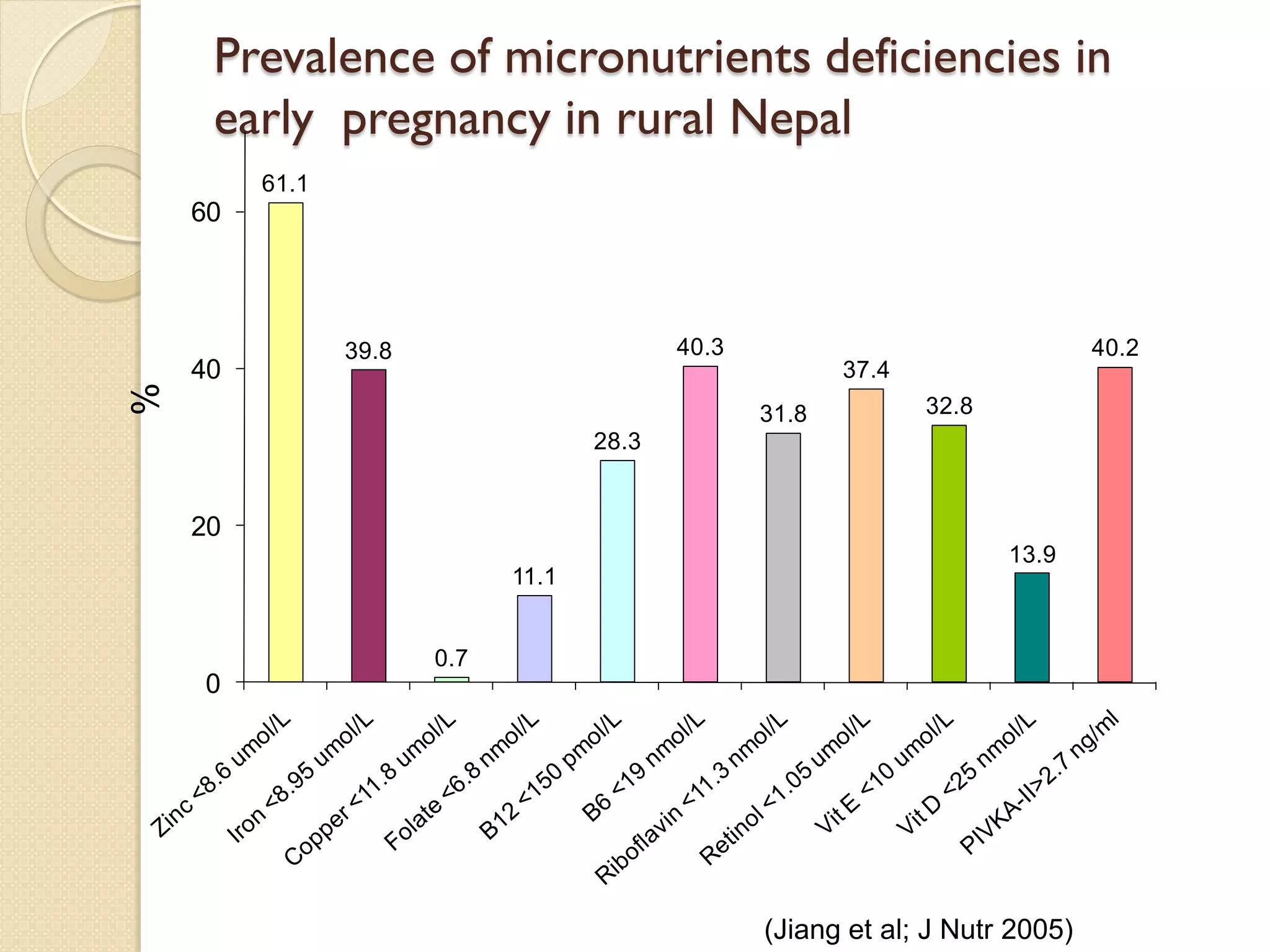
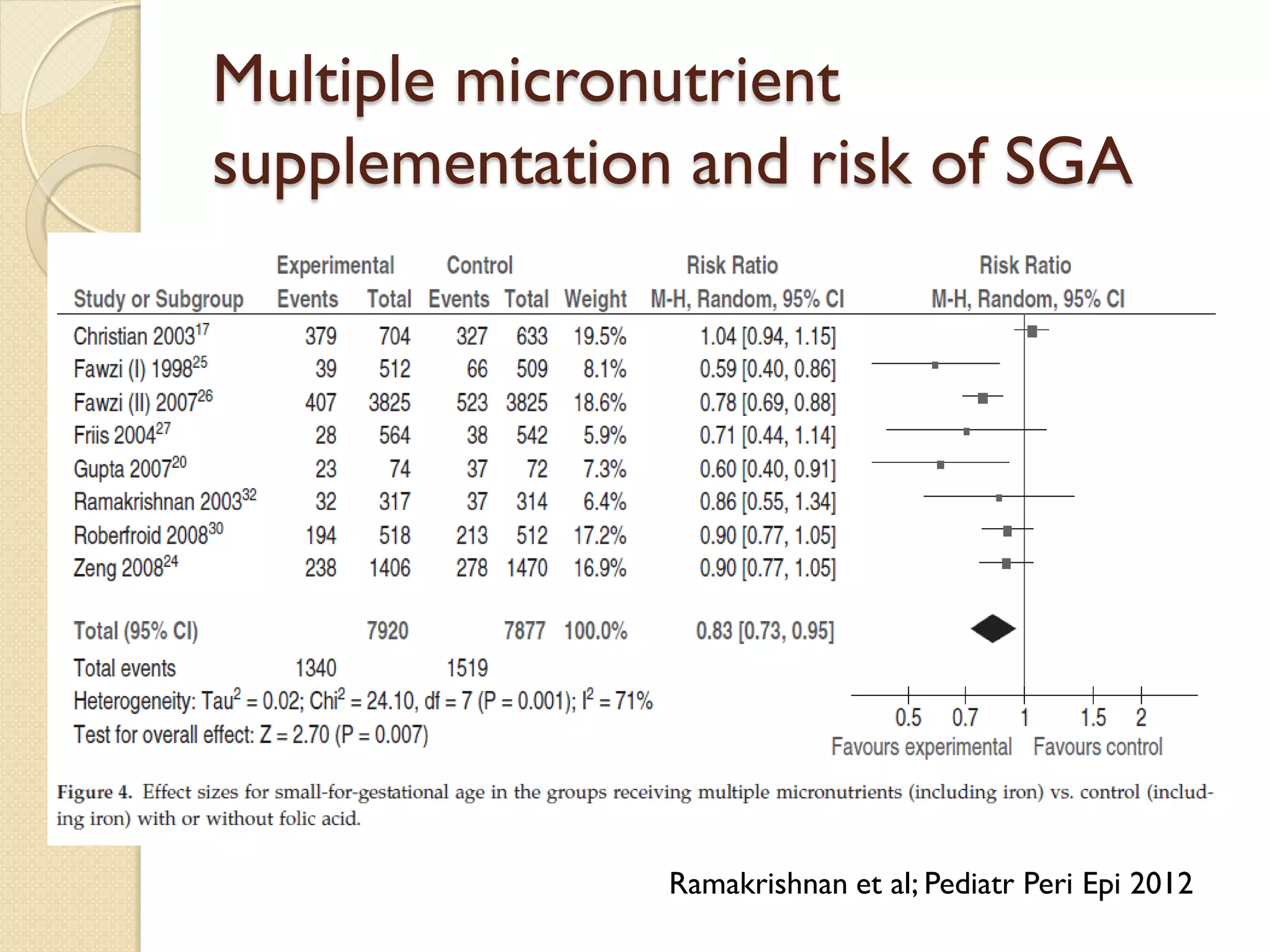
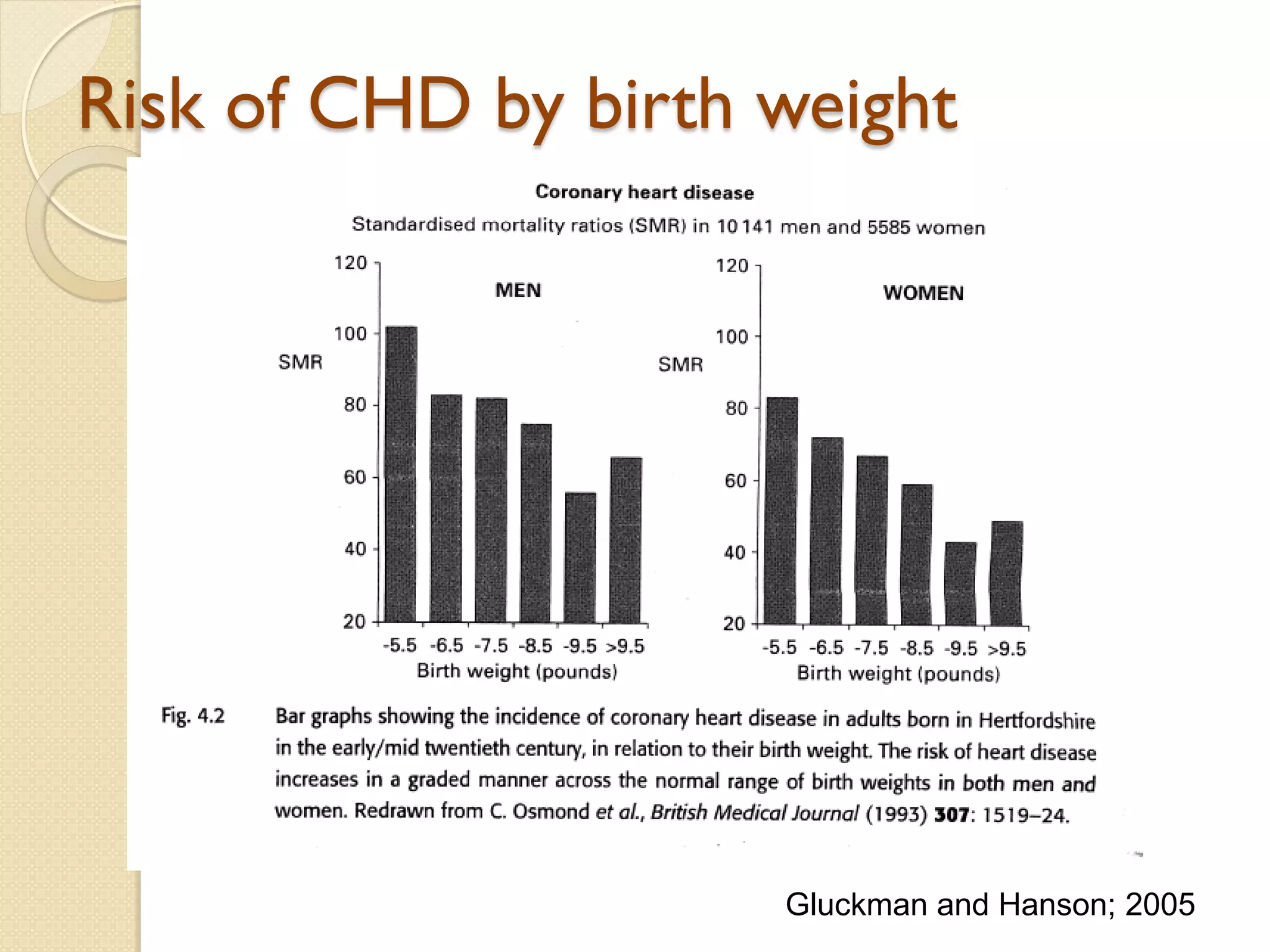
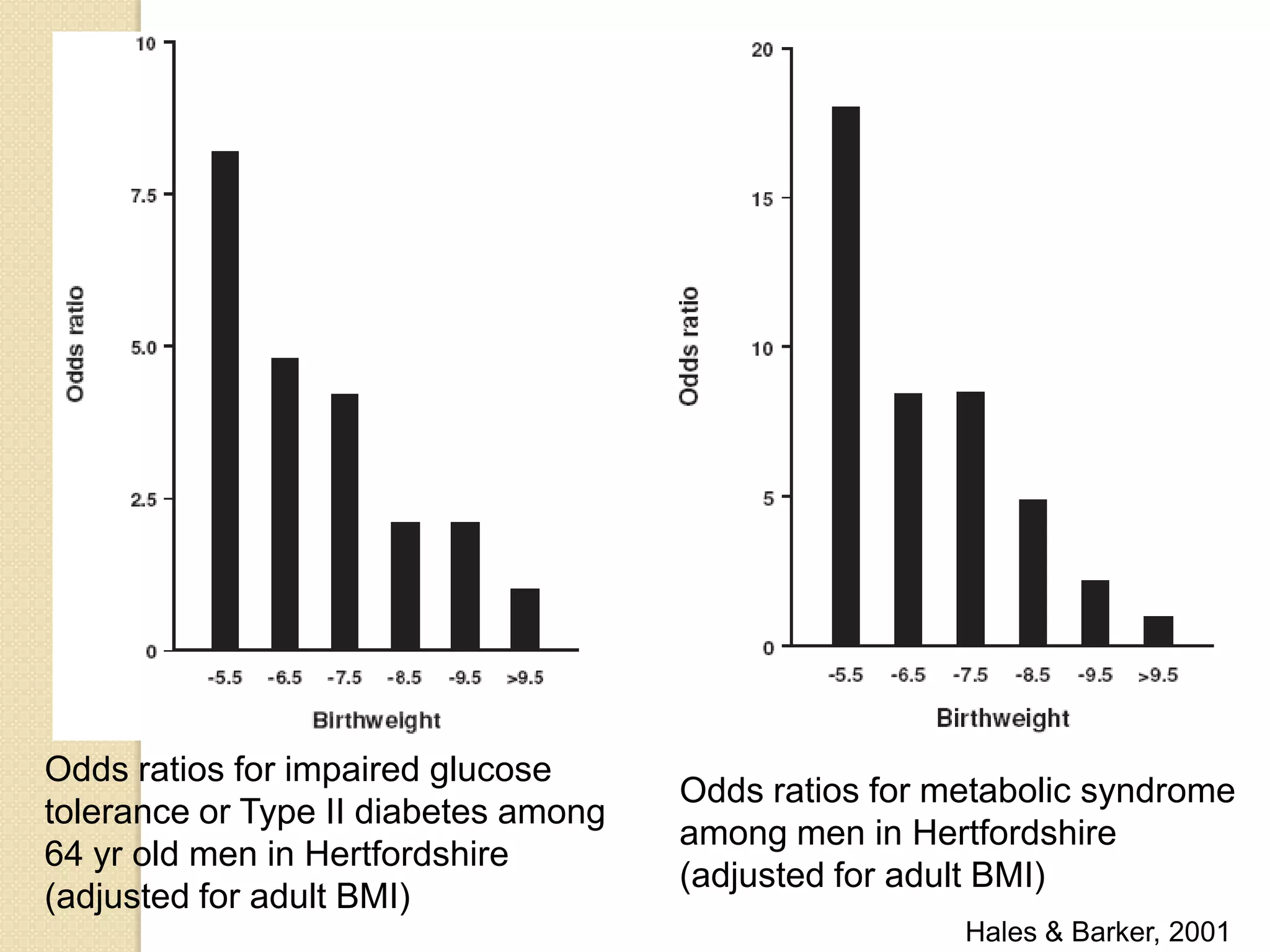
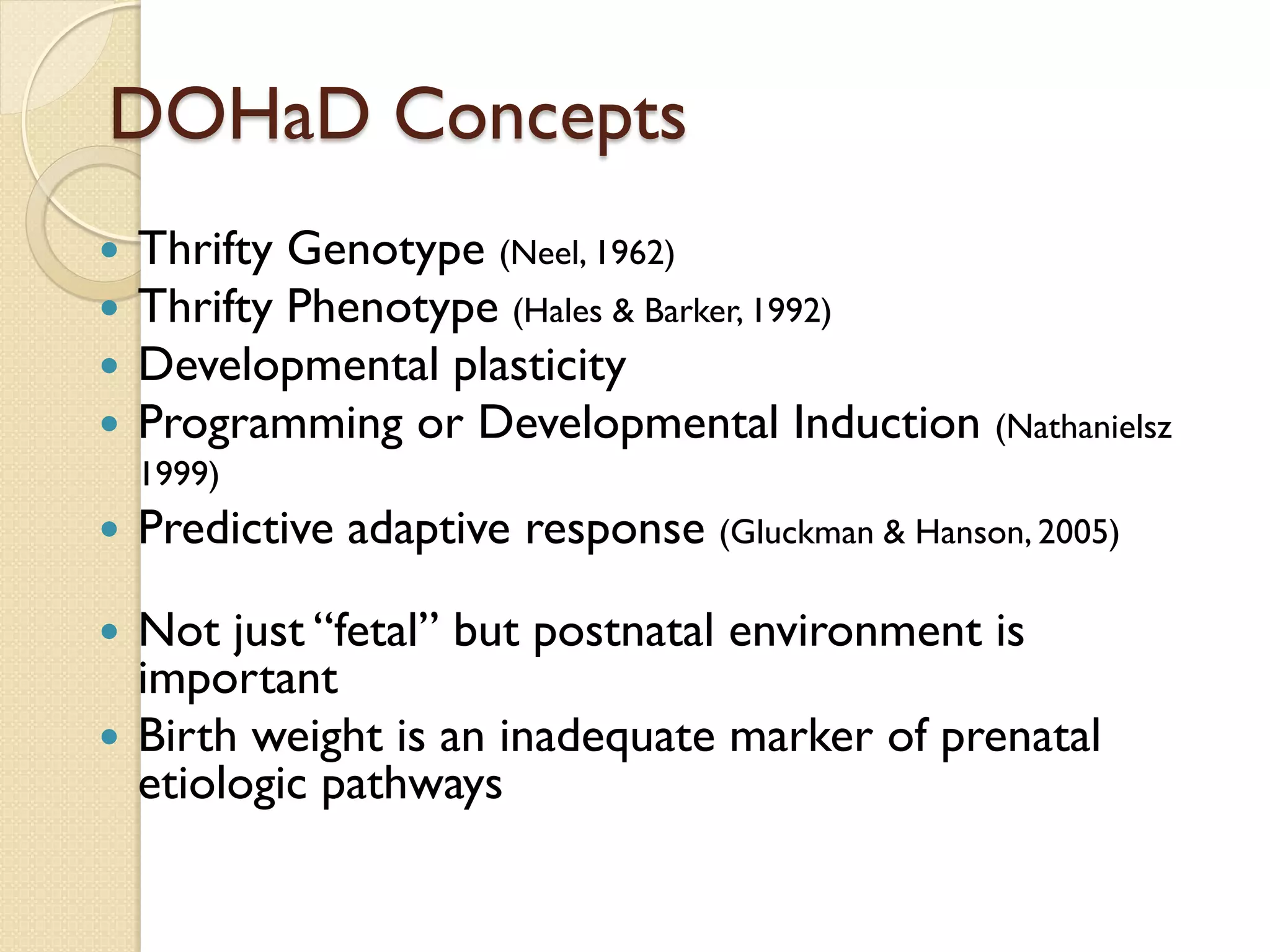
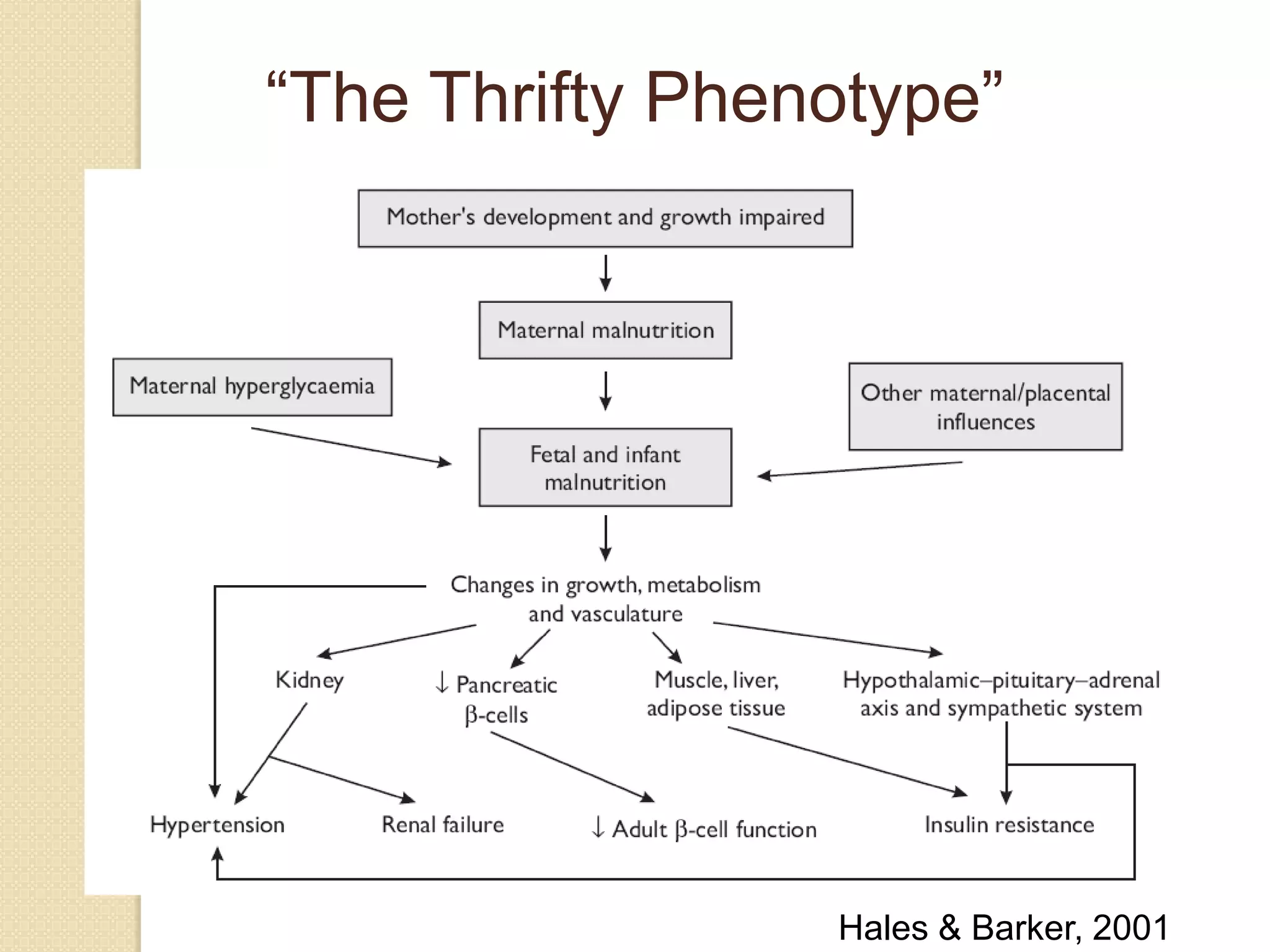
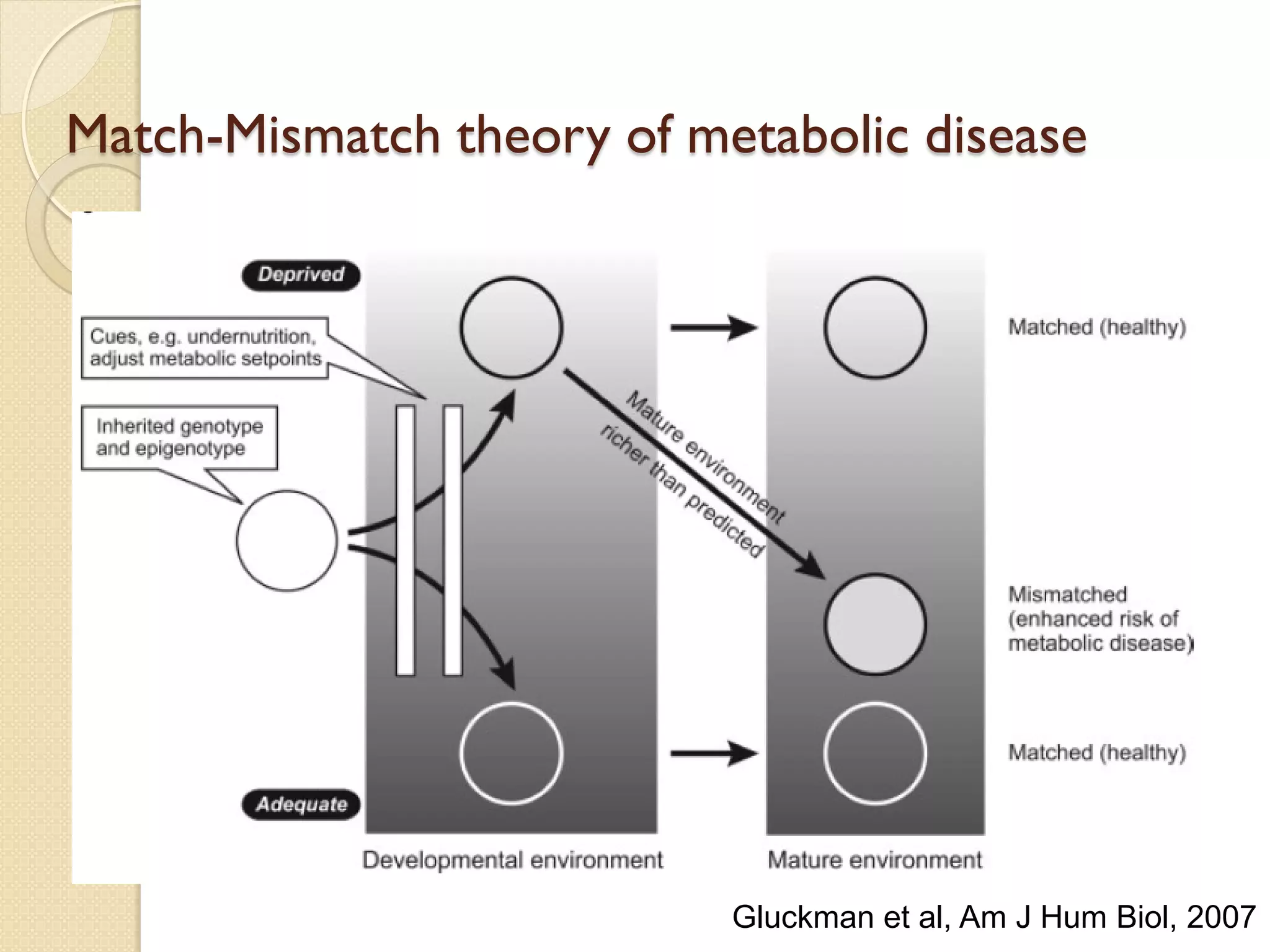
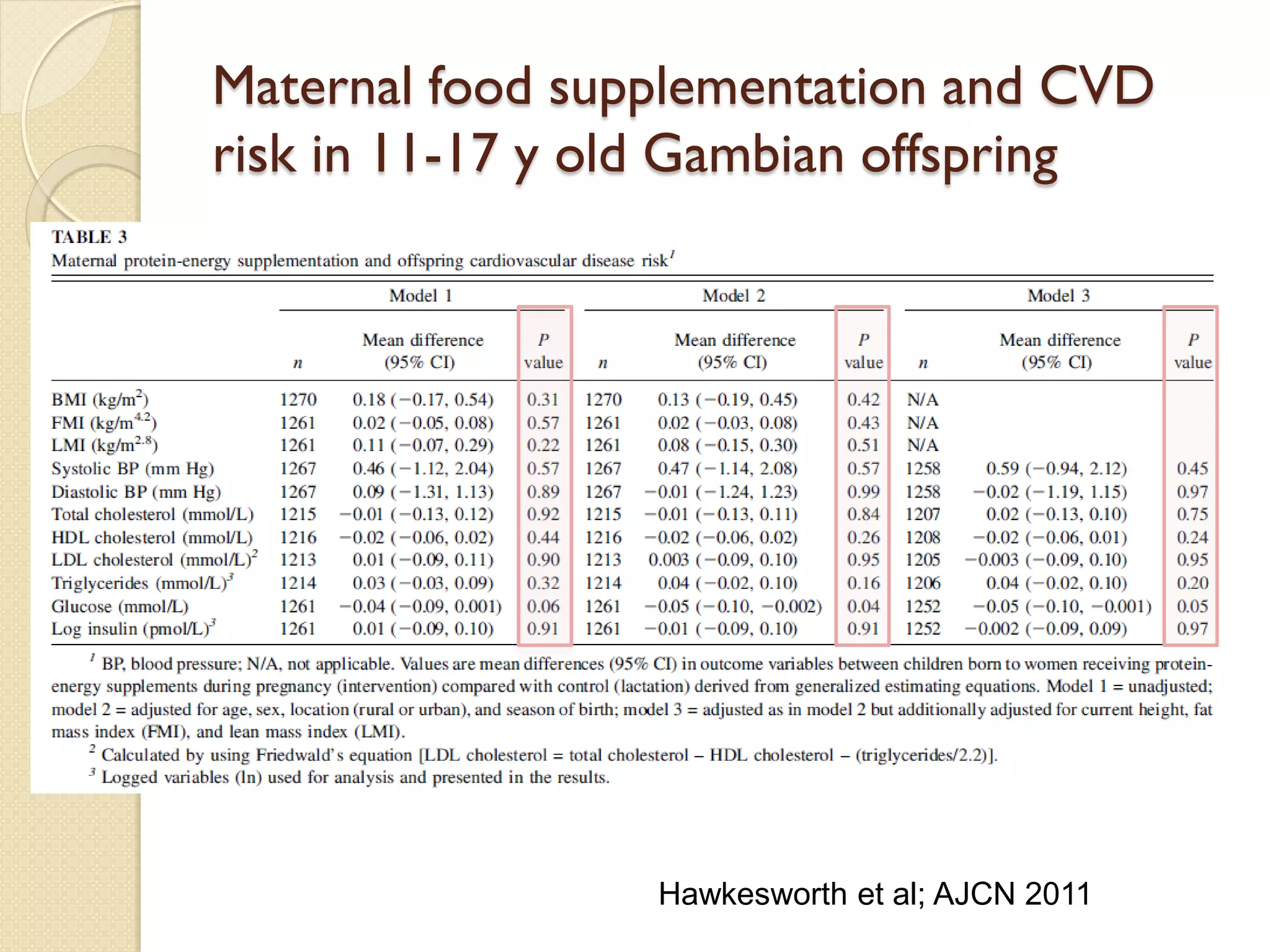
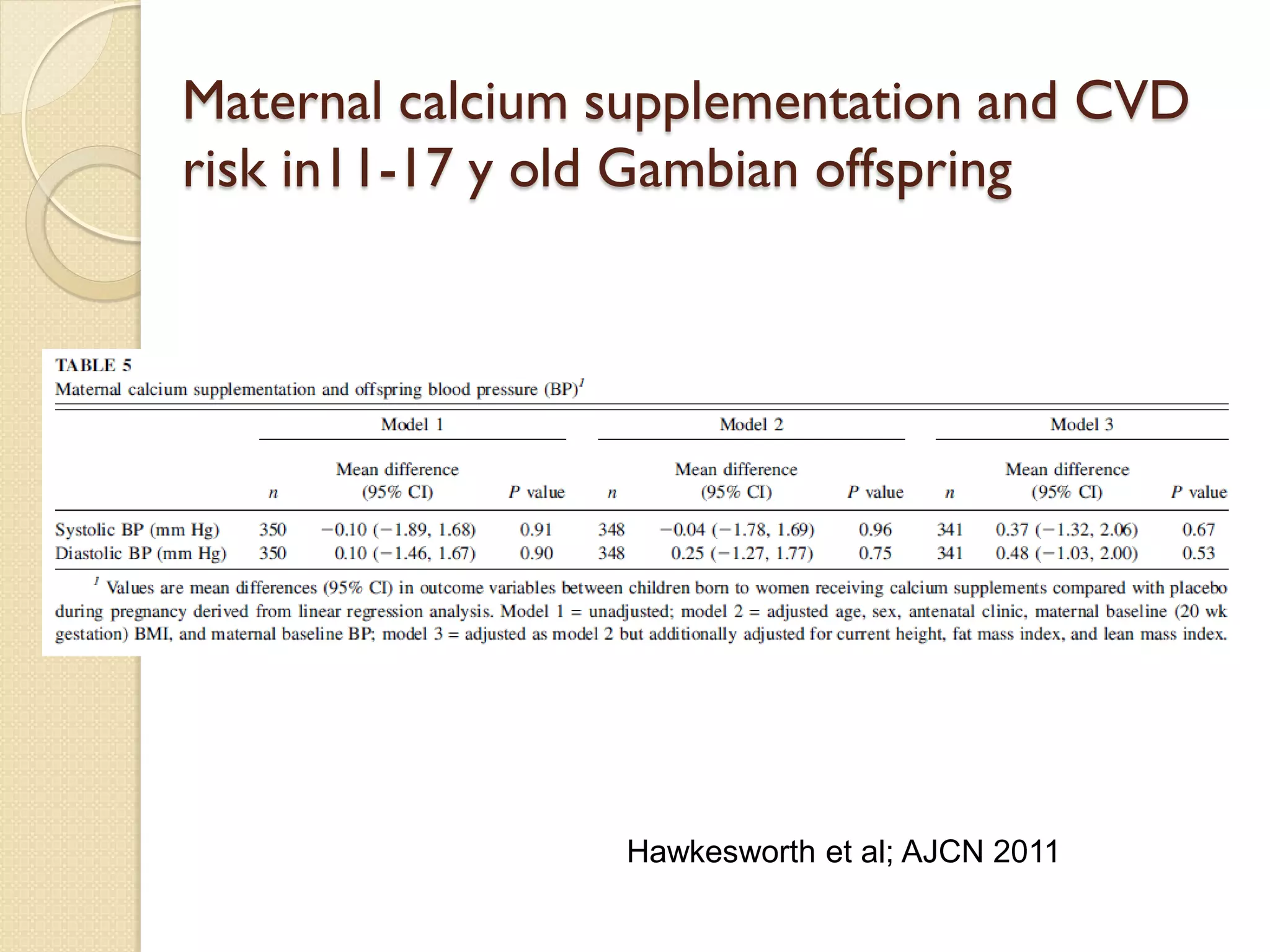
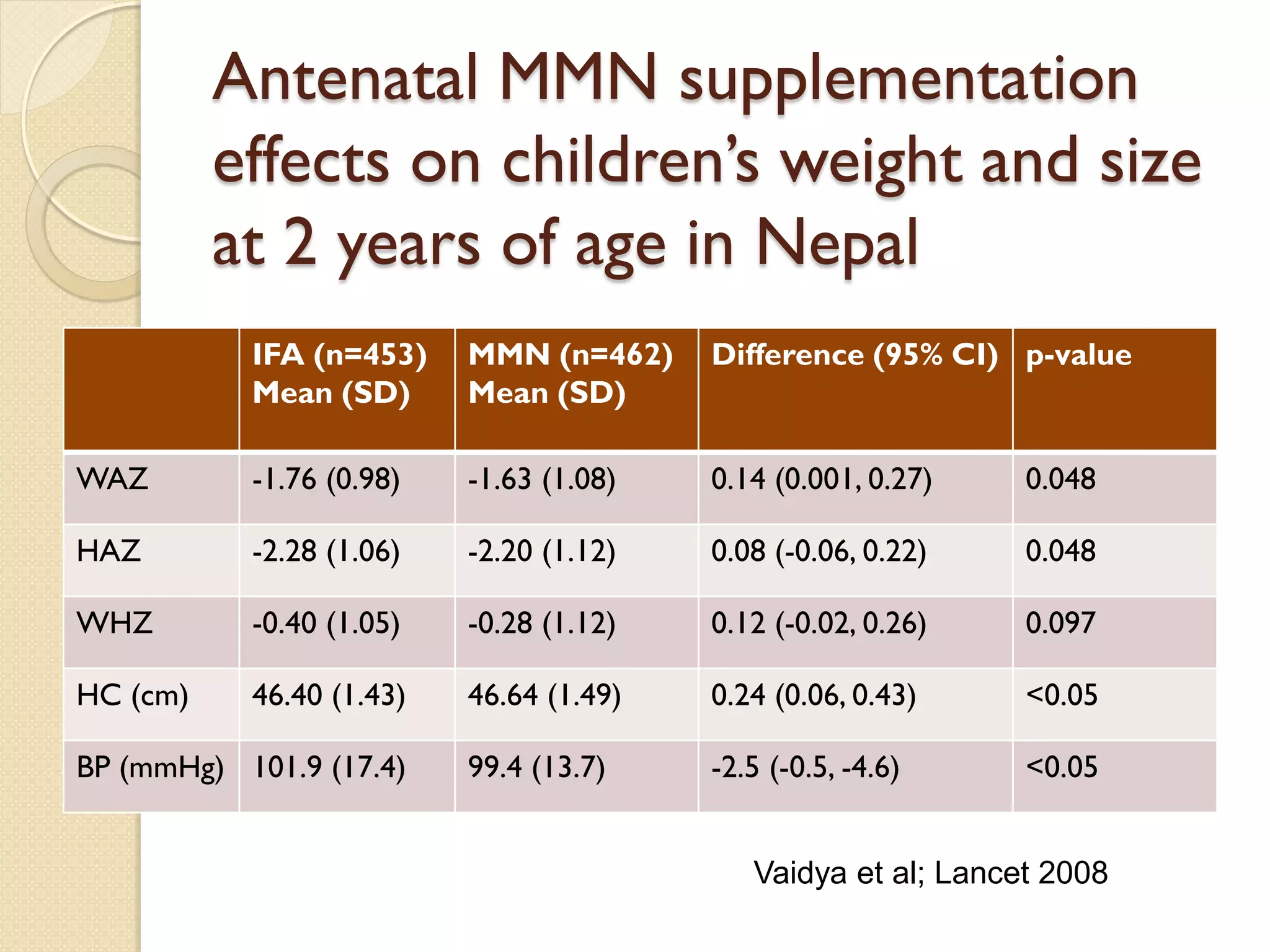
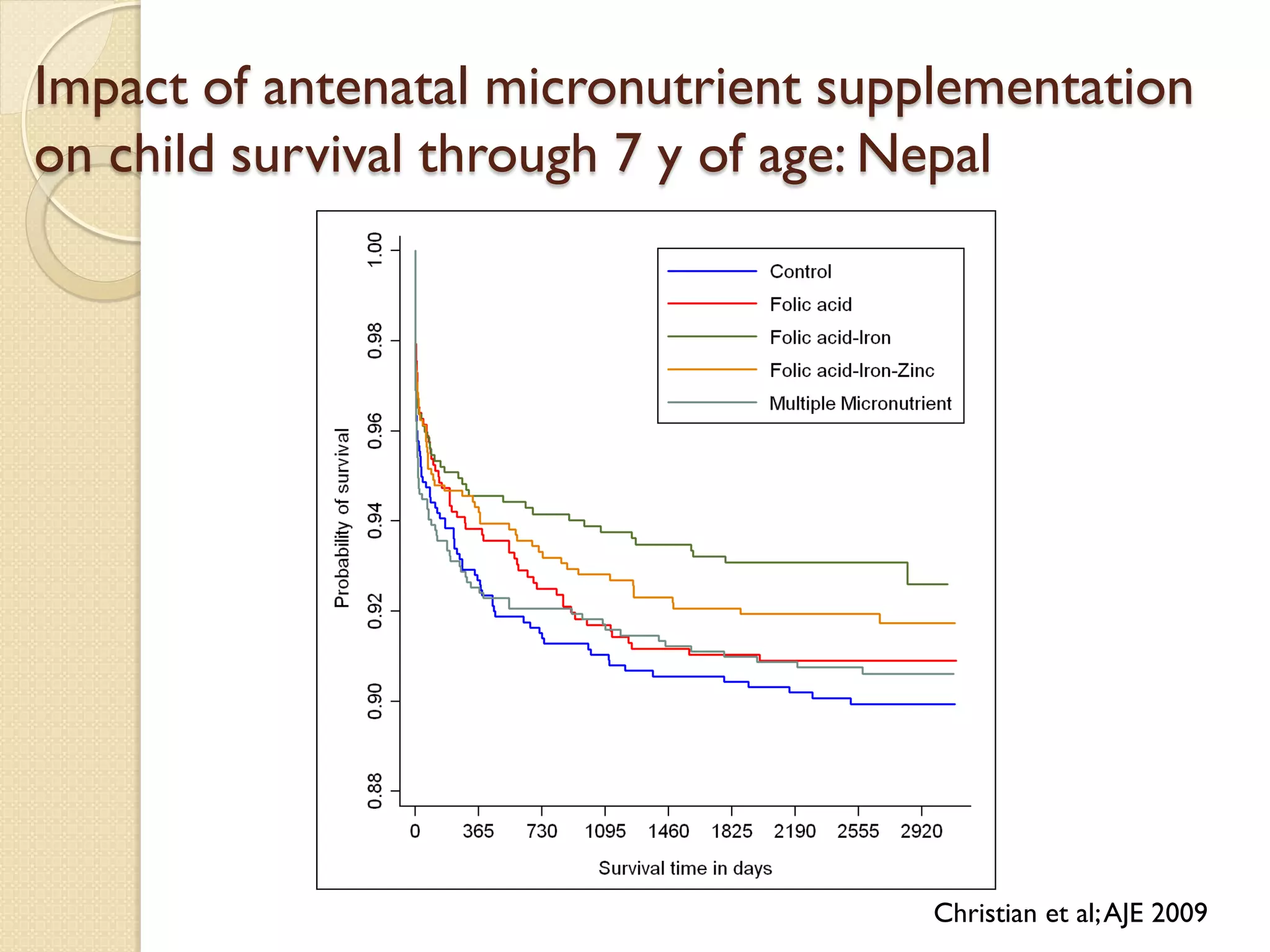
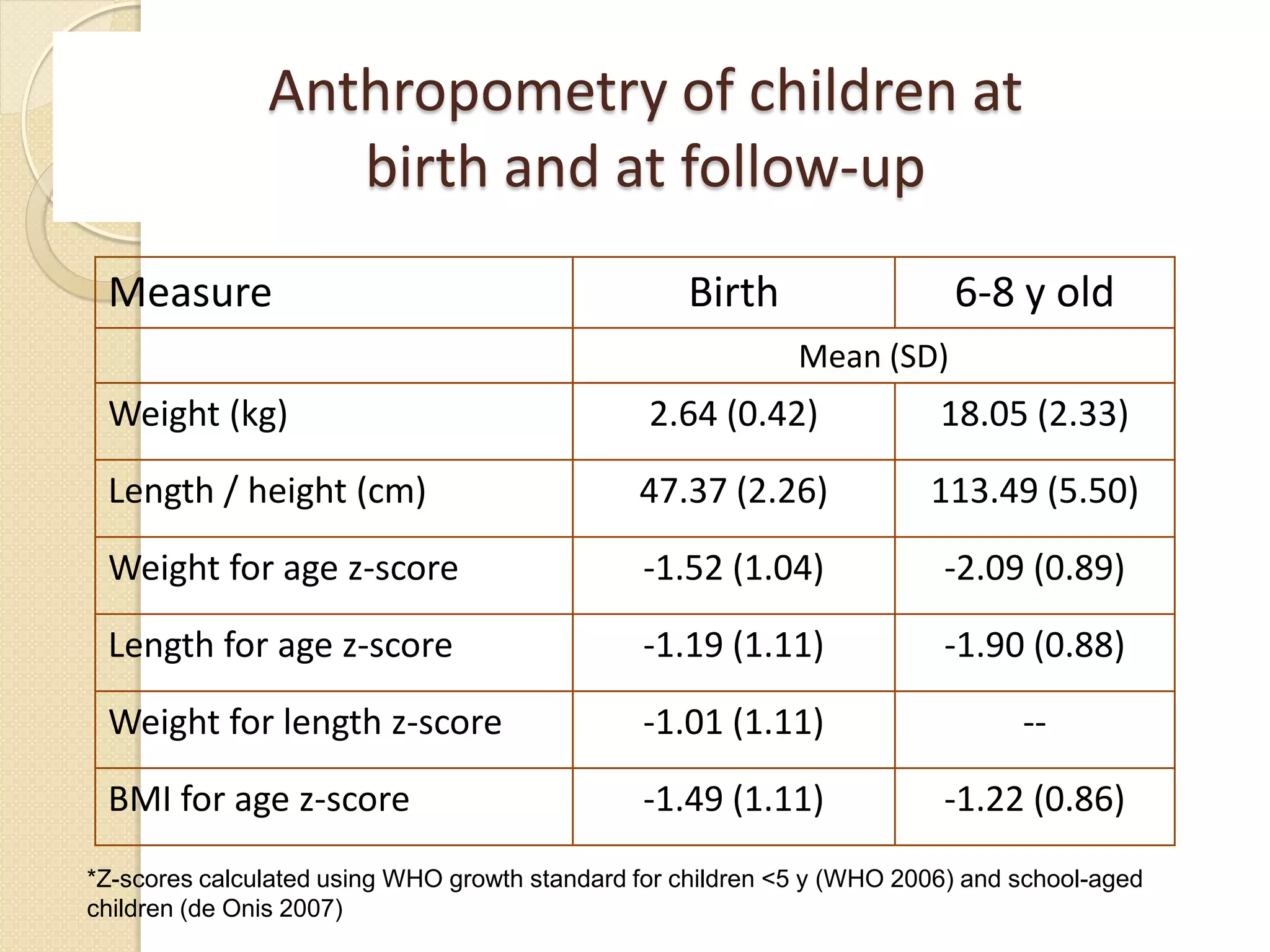
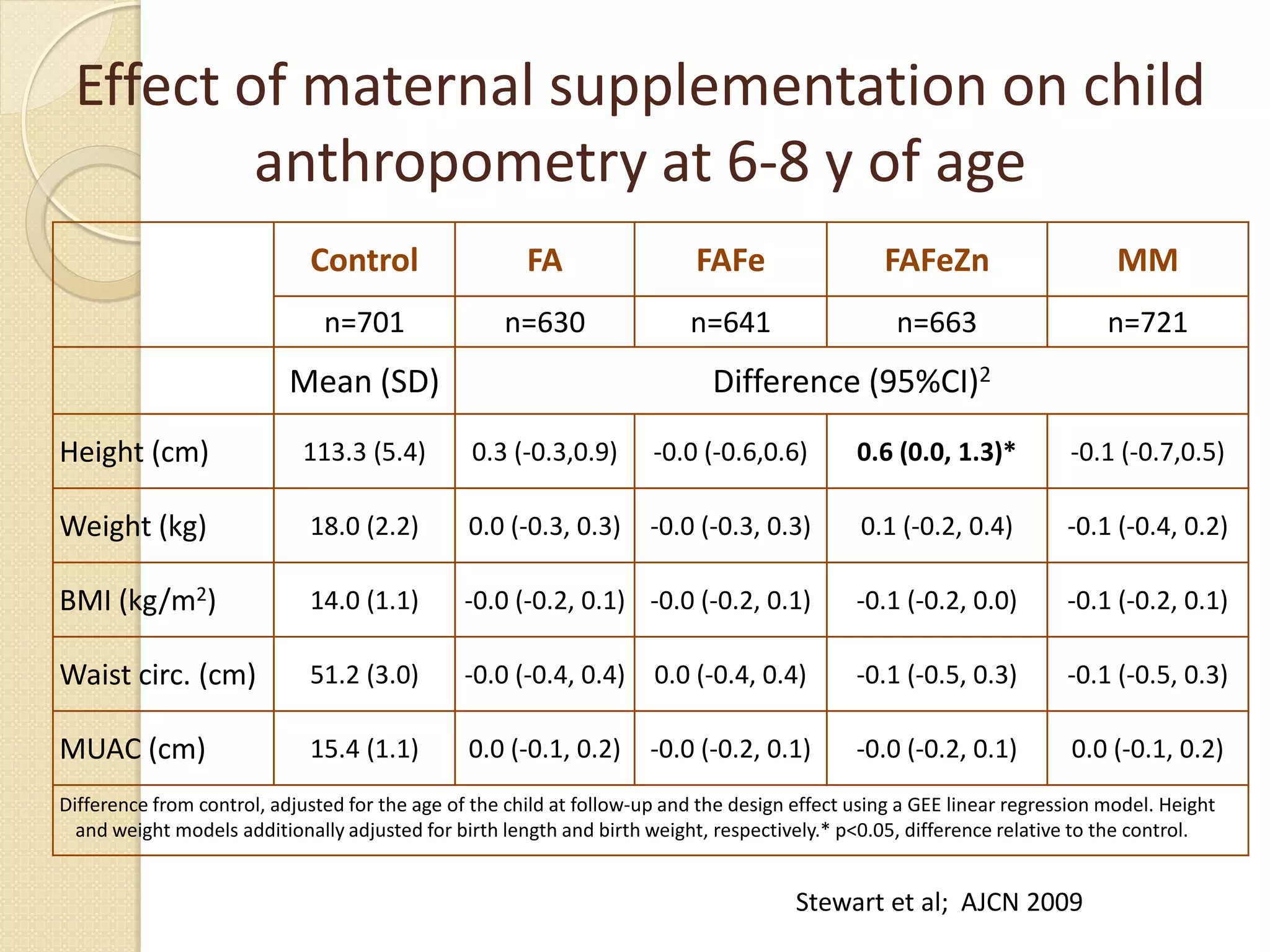
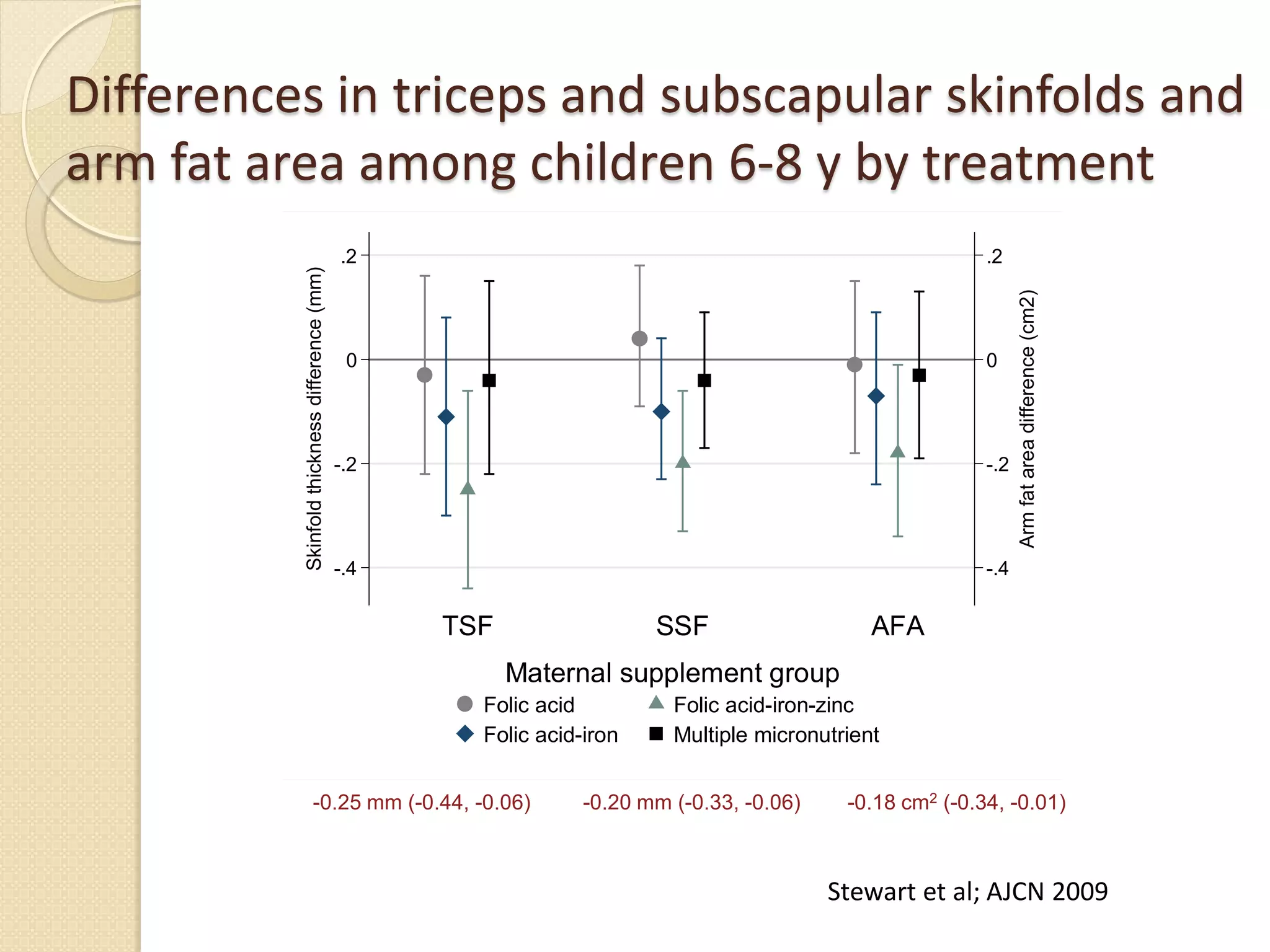
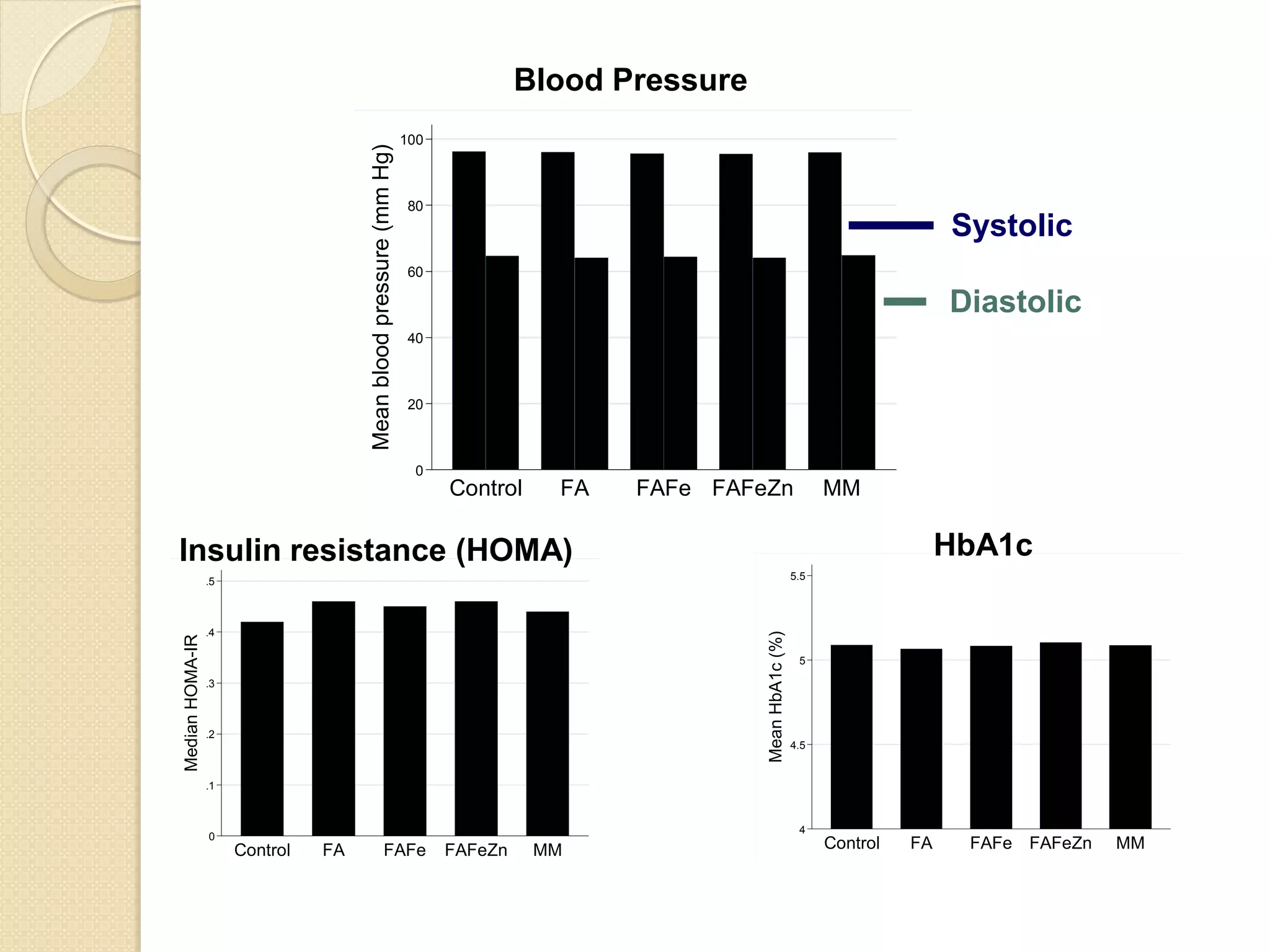
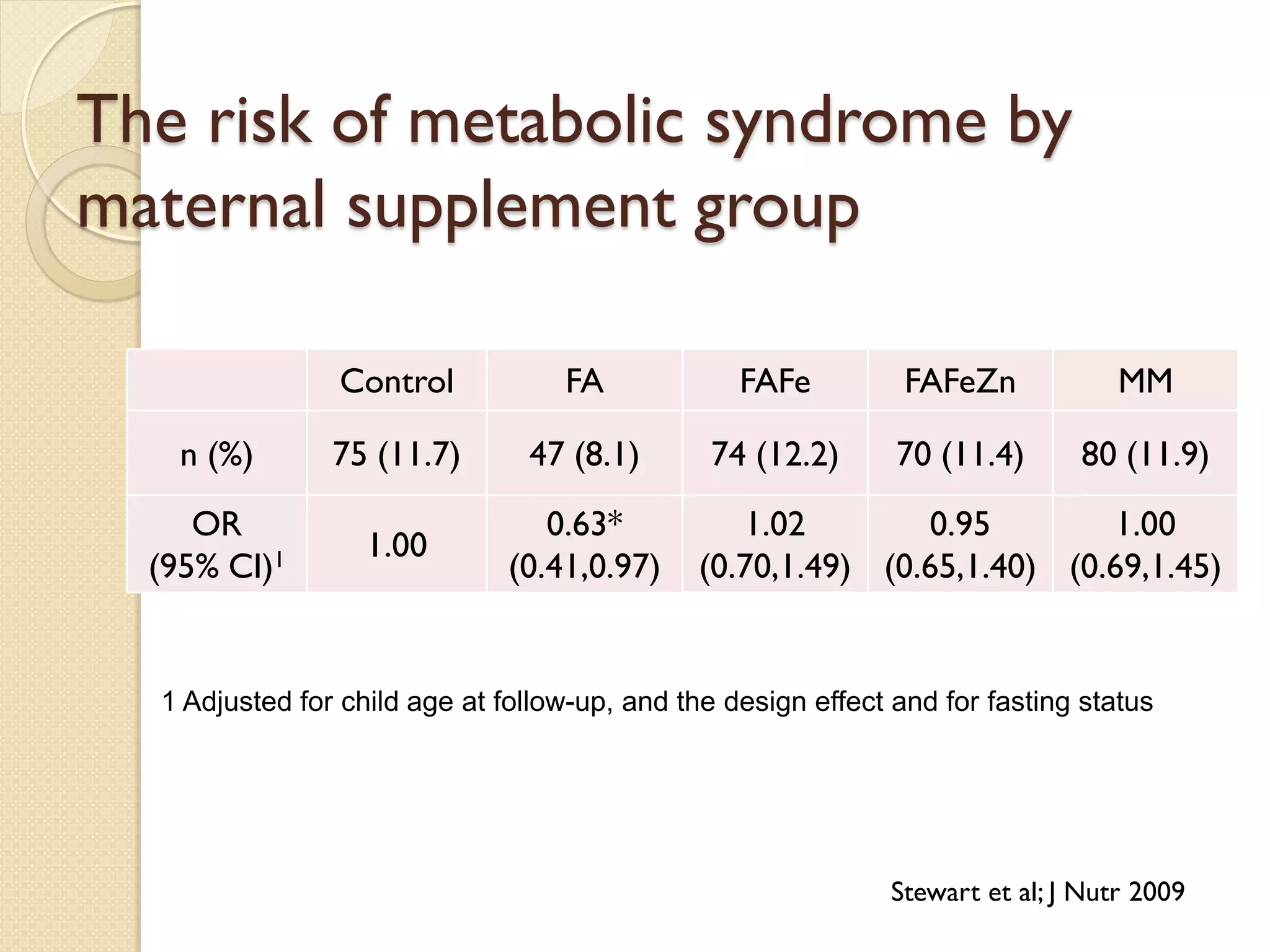
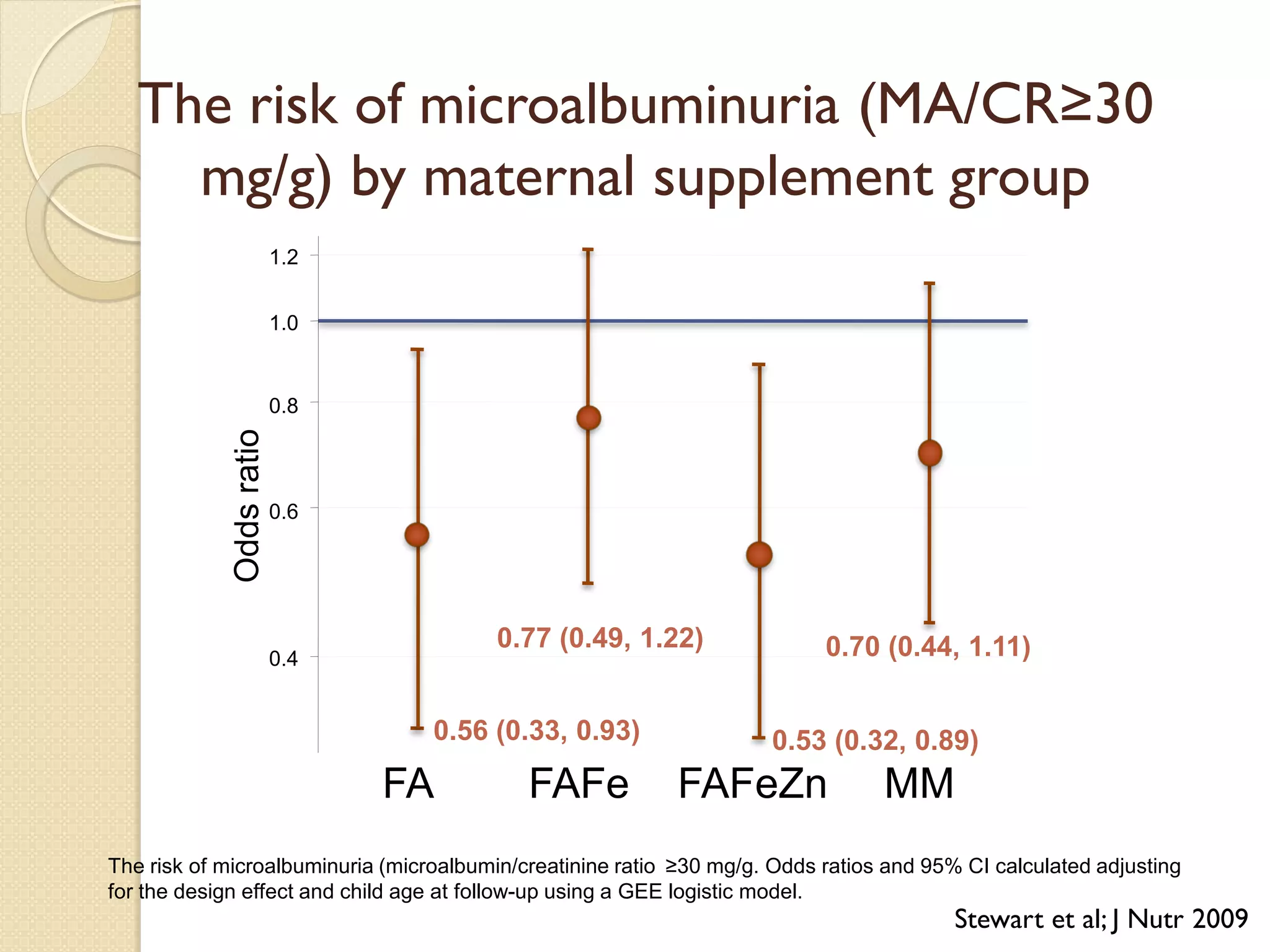
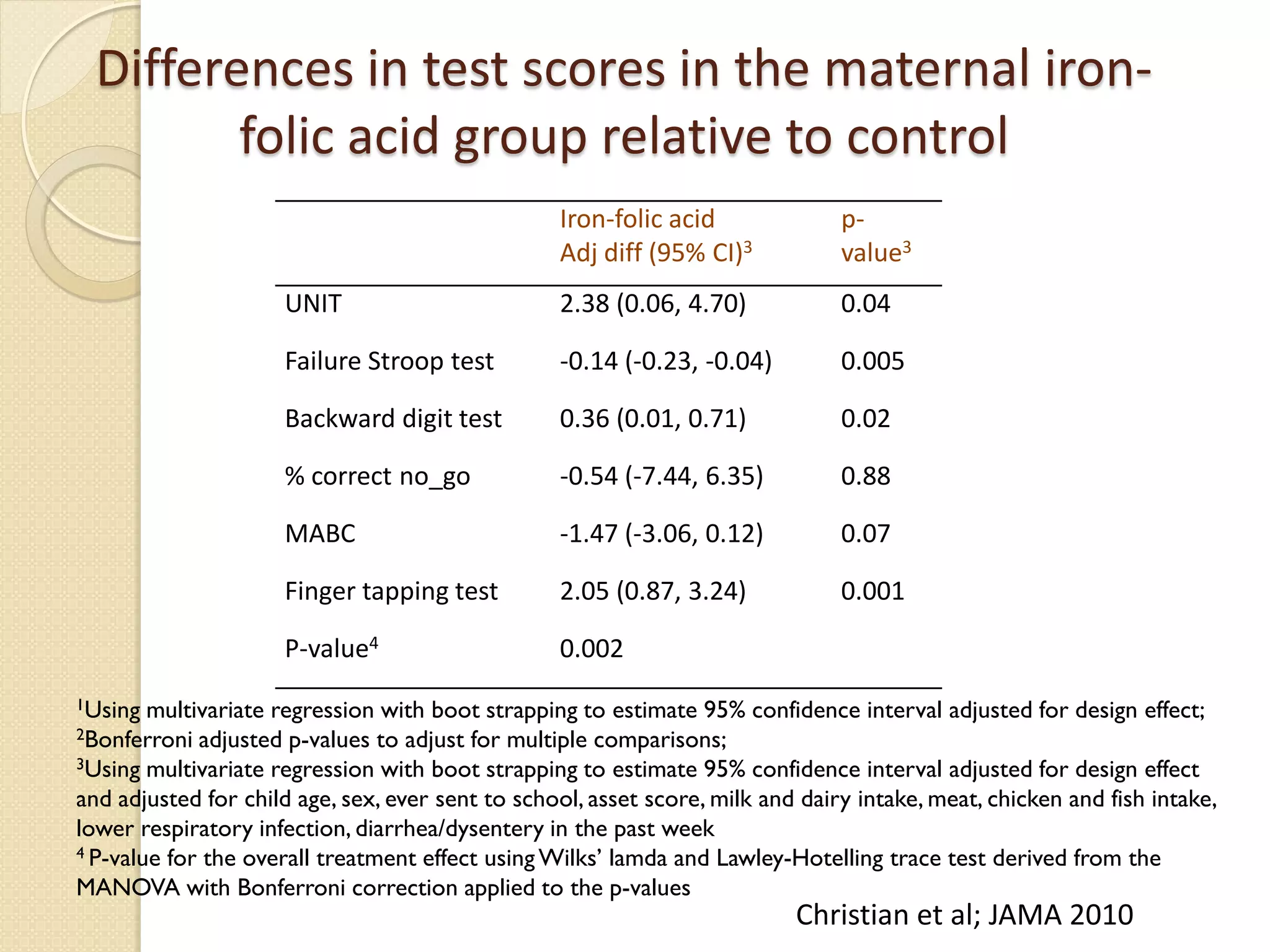
This document summarizes the results of a study on the impact of maternal nutritional interventions on child health outcomes. It finds that interventions like food and micronutrient supplementation can positively impact birth weight and fetal growth. While evidence on effects on gestational age is limited, supplementation is shown to reduce the risk of low birth weight and small-for-gestational-age babies. The study also finds long term effects on child growth, cognition, and risk of metabolic syndrome from maternal supplementation. Further research is still needed to better understand impacts of preconception interventions and the importance of a life-course approach.