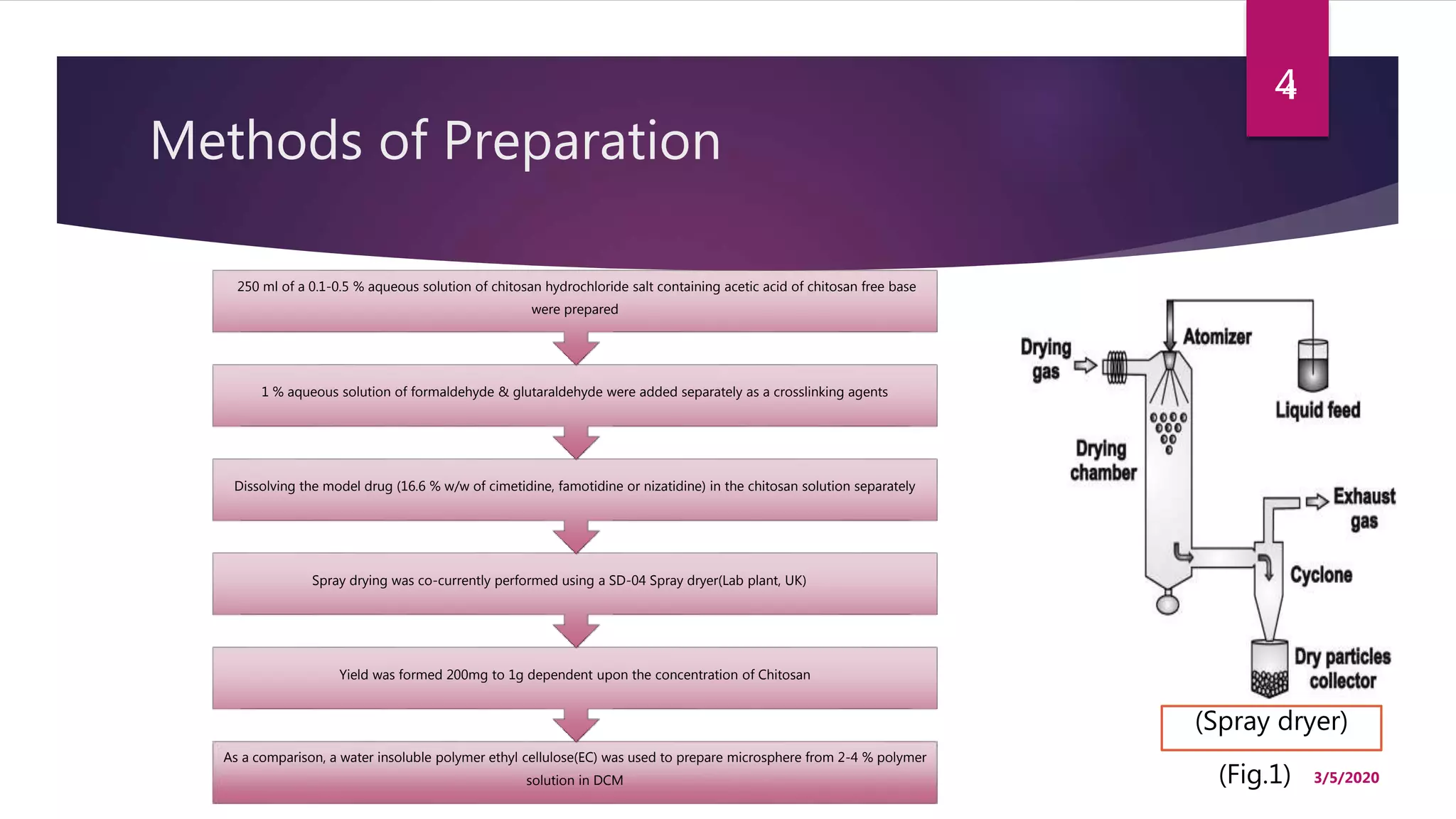
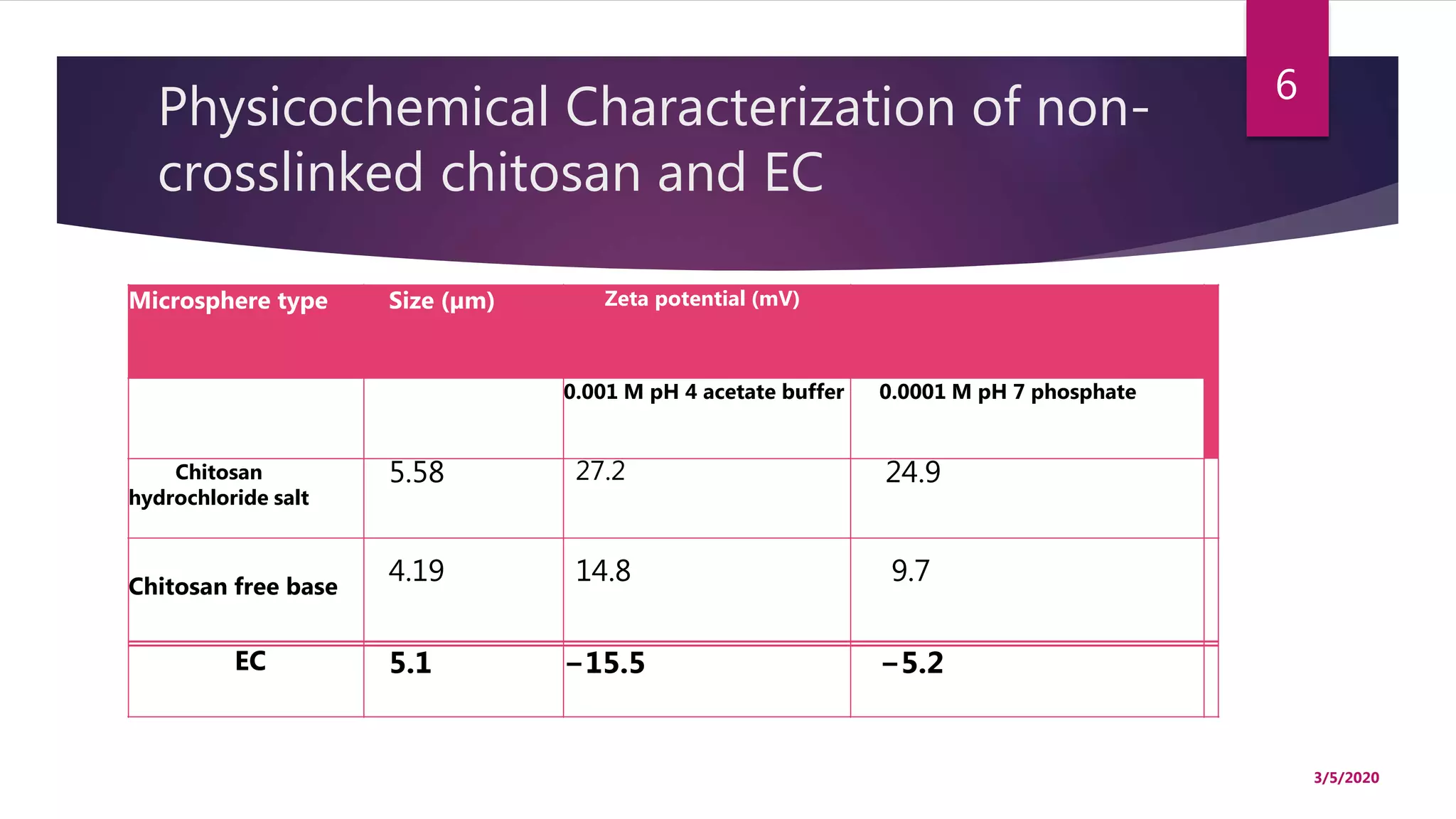
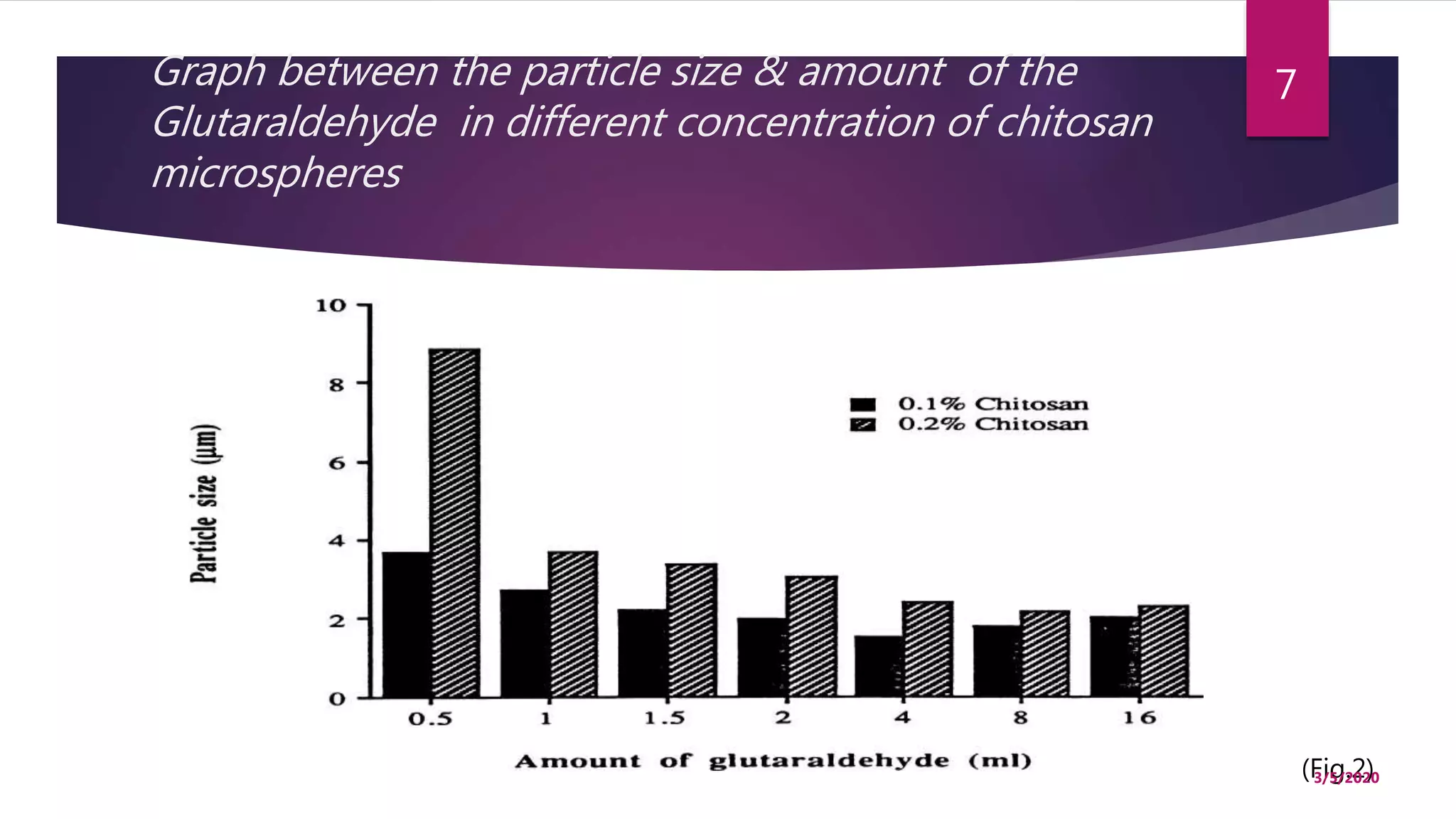
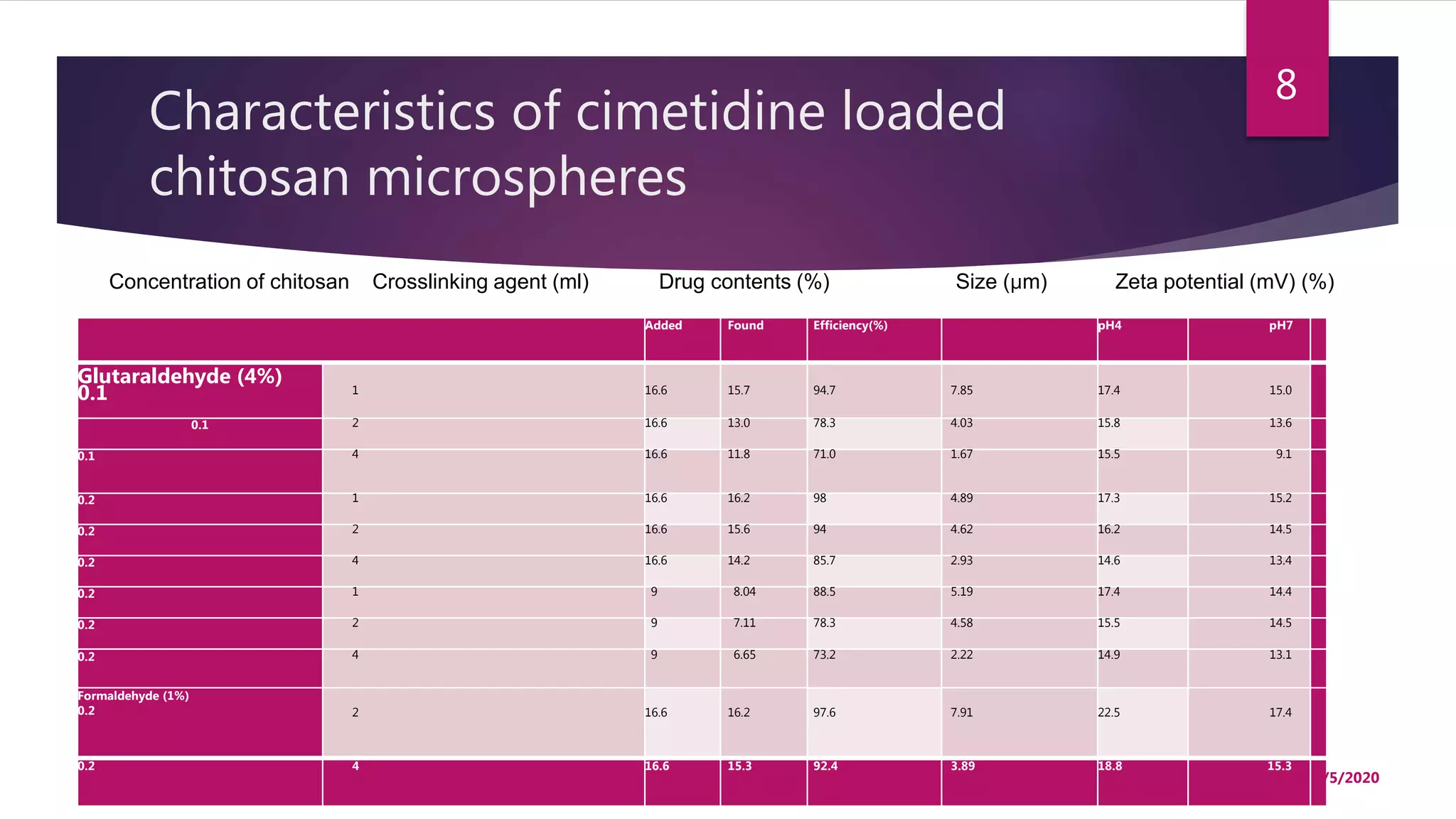
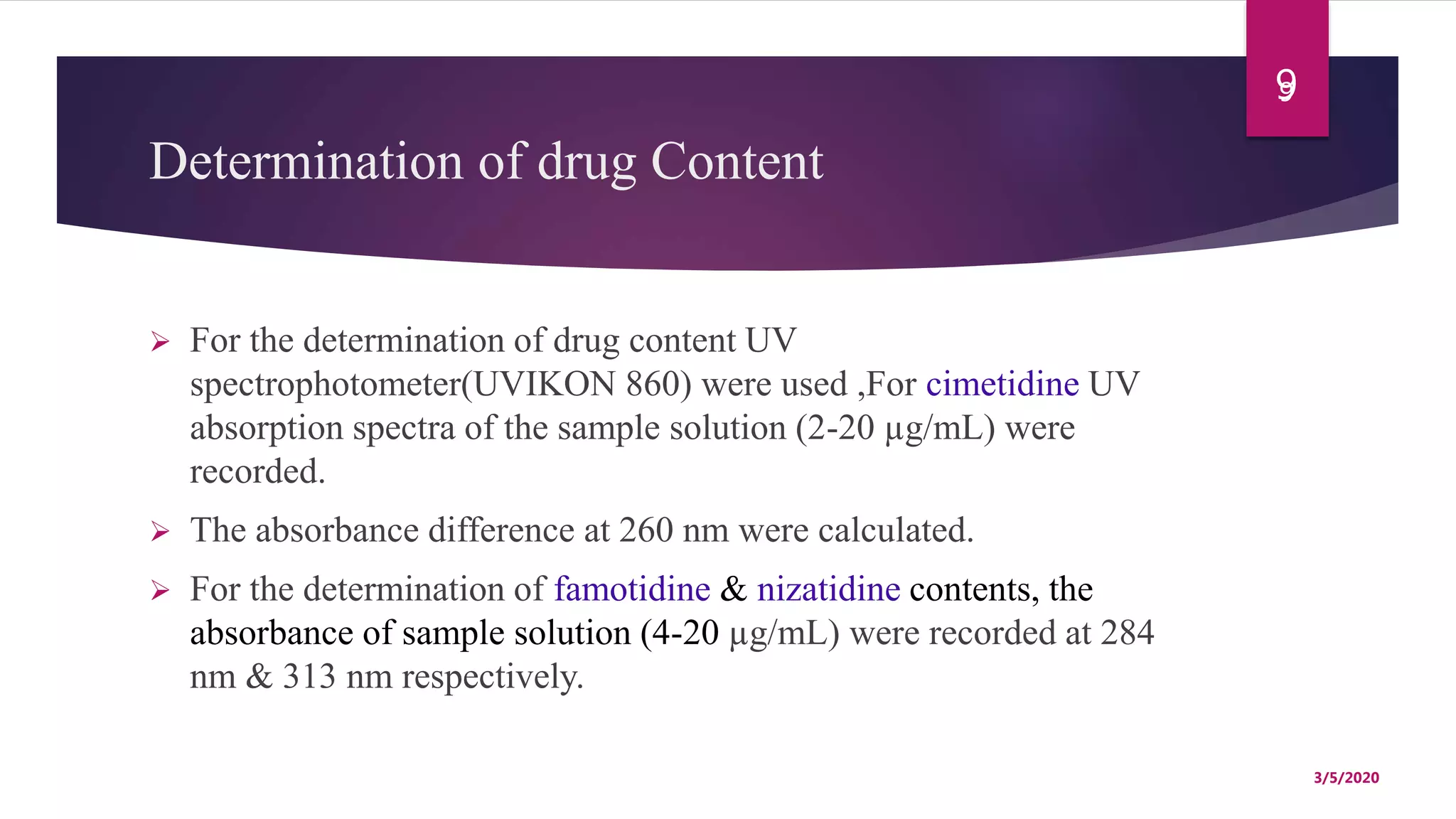
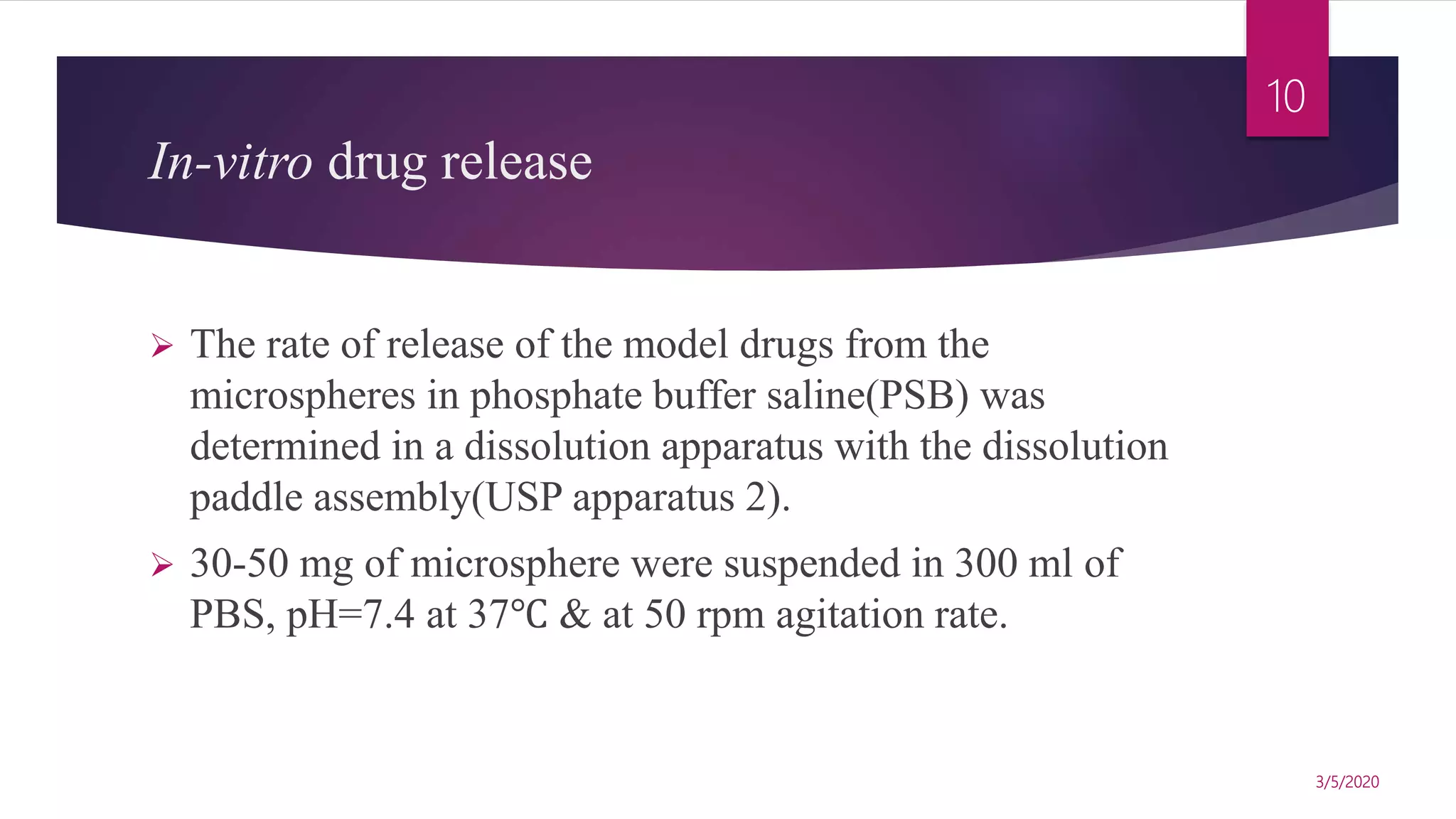
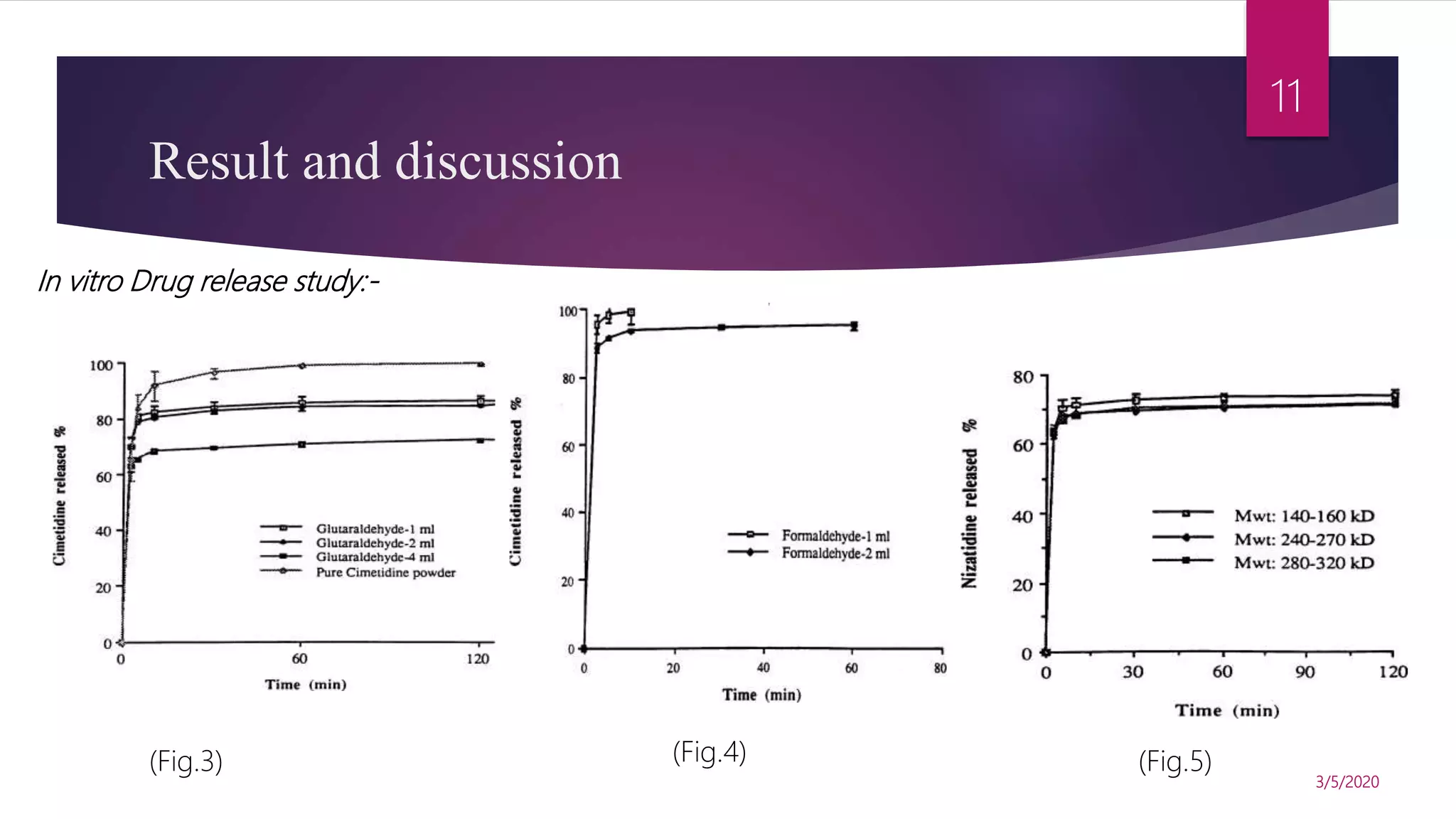
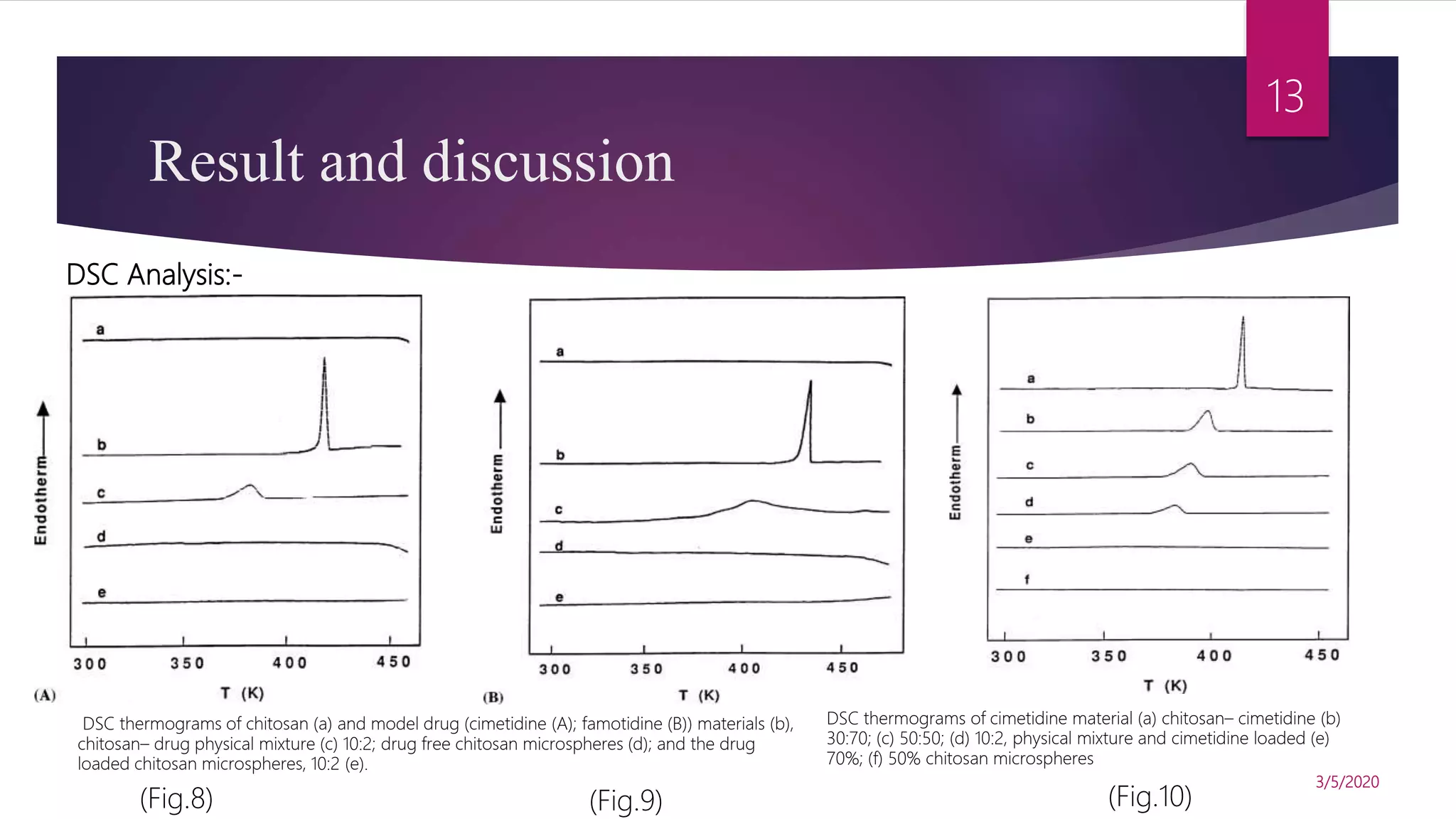
Chitosan microspheres were prepared using a spray drying method. Non-crosslinked and crosslinked microspheres were created using glutaraldehyde or formaldehyde as crosslinking agents. The microspheres ranged in size from 2-10 μm, were spherical and smooth, and had a positive surface charge. Model drugs including cimetidine, famotidine, and nizatidine were loaded into the microspheres at various concentrations. Drug release studies showed an initial burst release followed by sustained release of the drugs from the microspheres over time. Scanning electron microscopy images showed the spherical morphology of the drug-loaded microspheres.