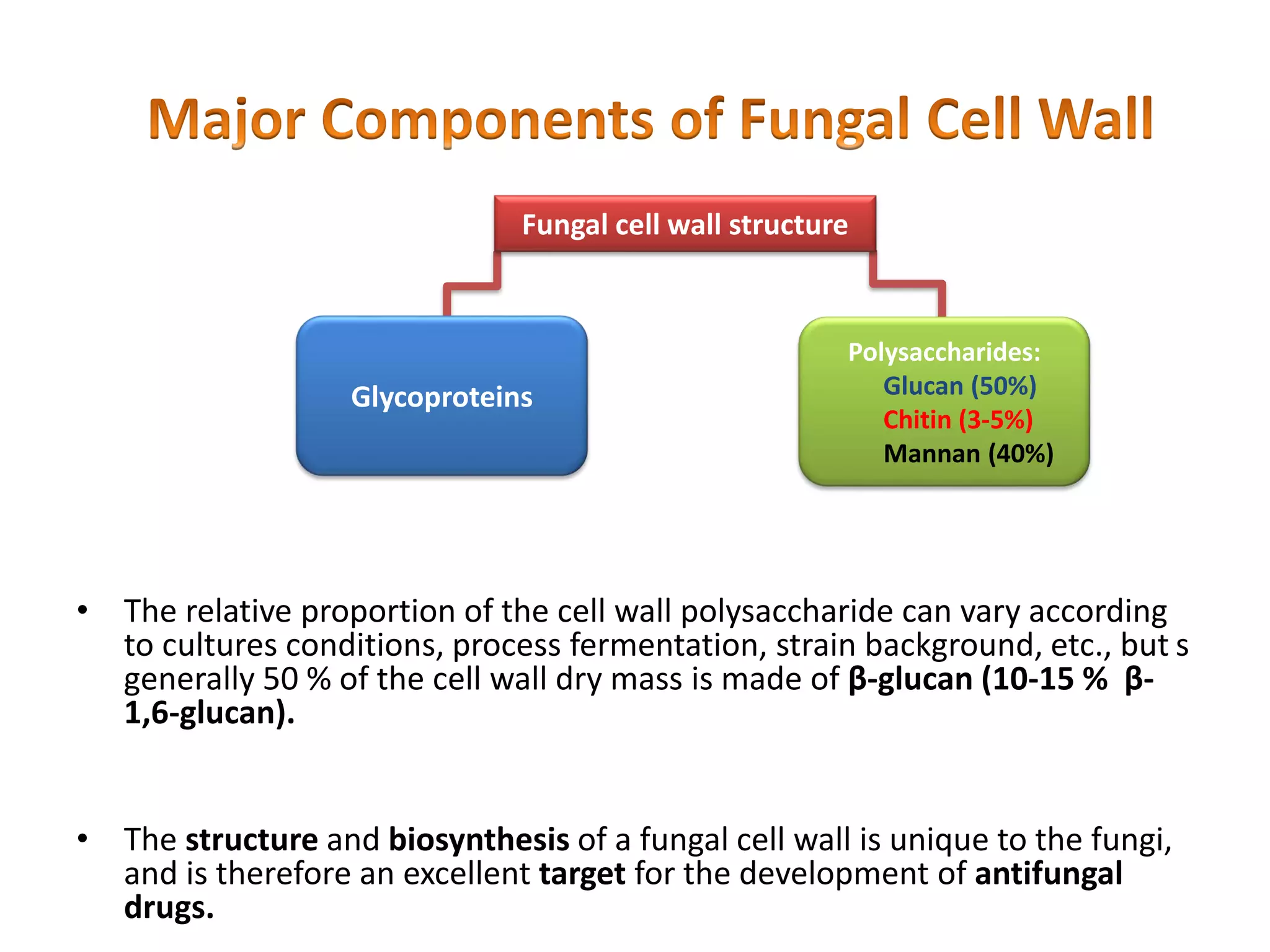
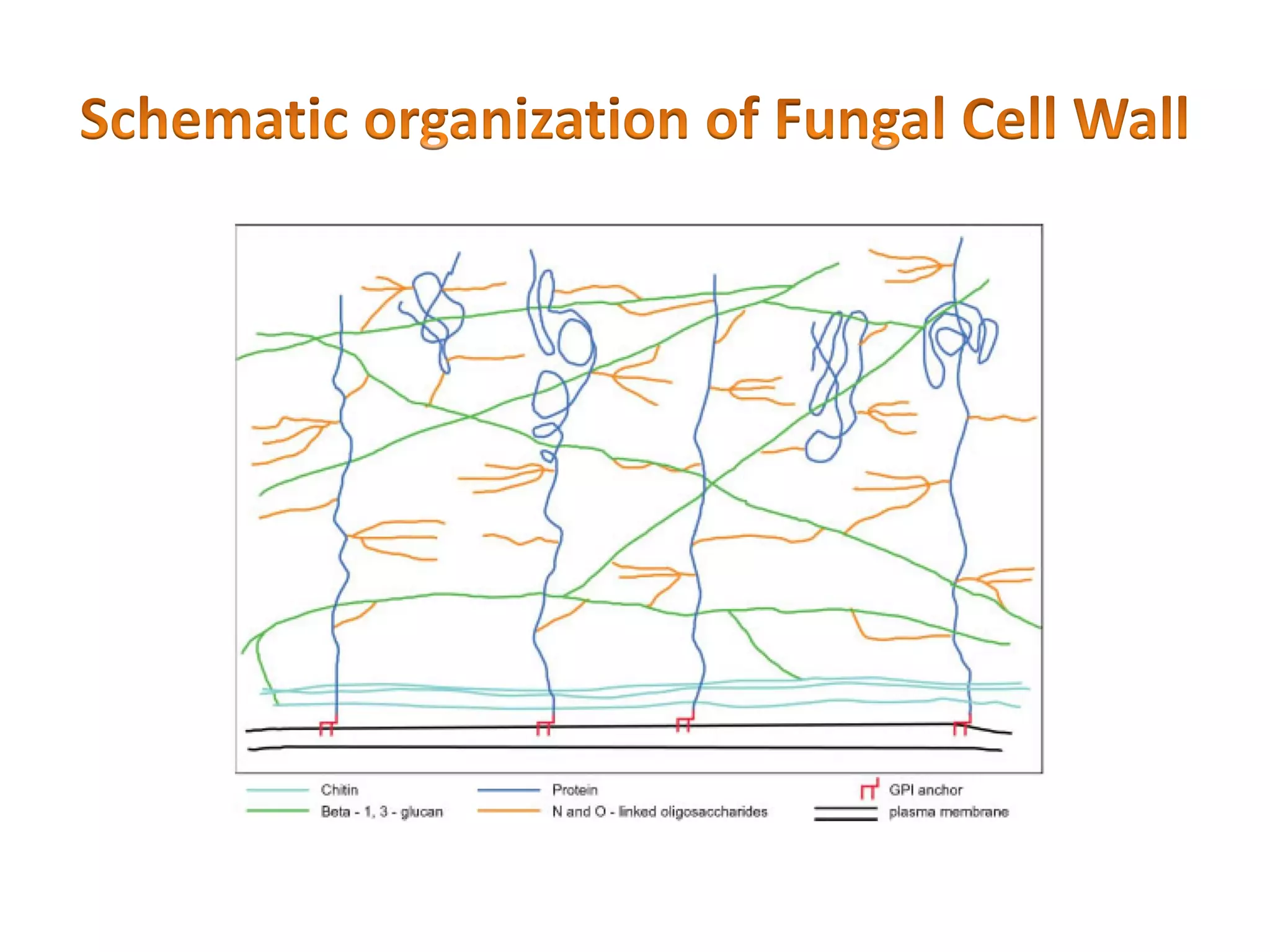
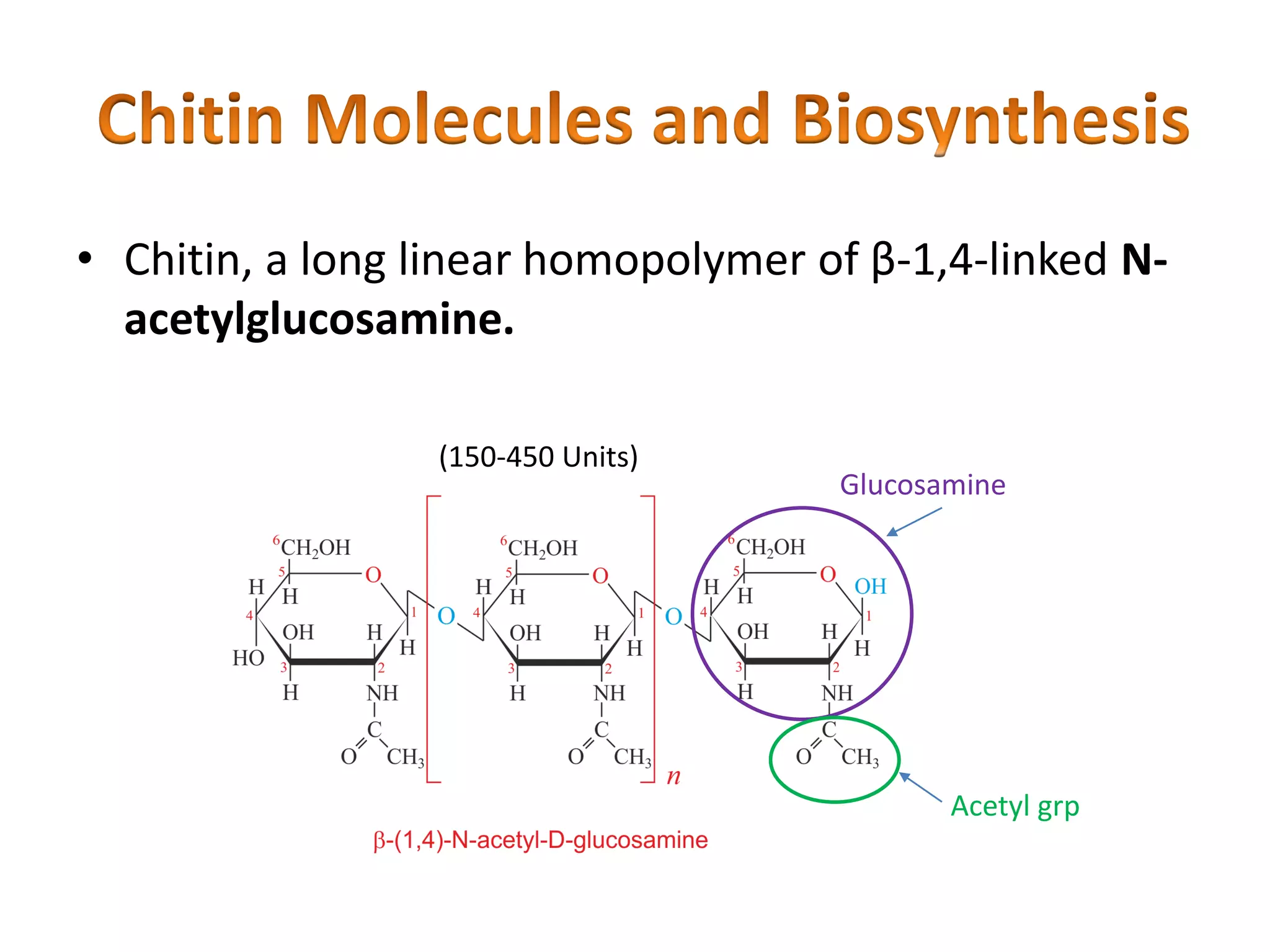
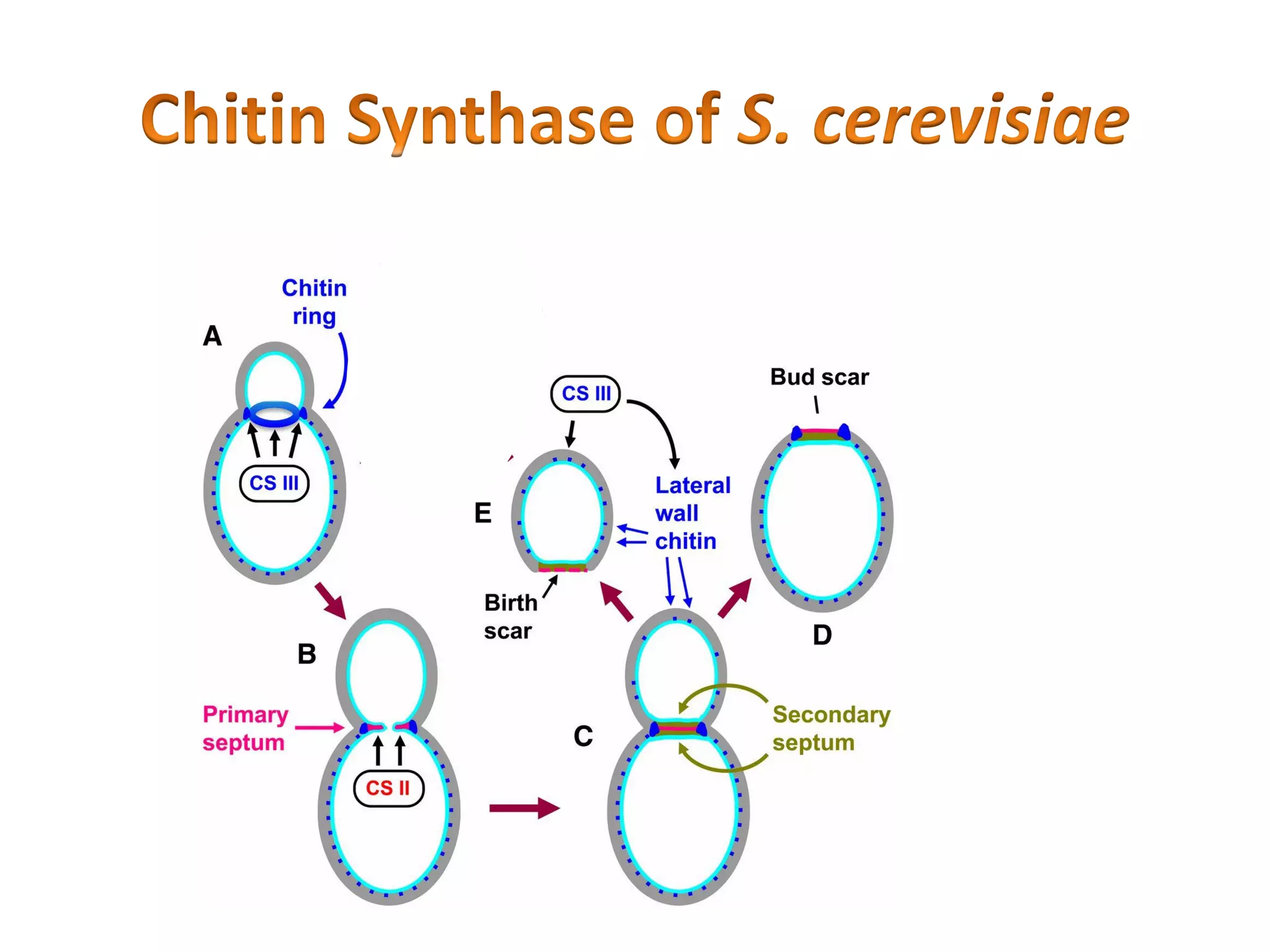
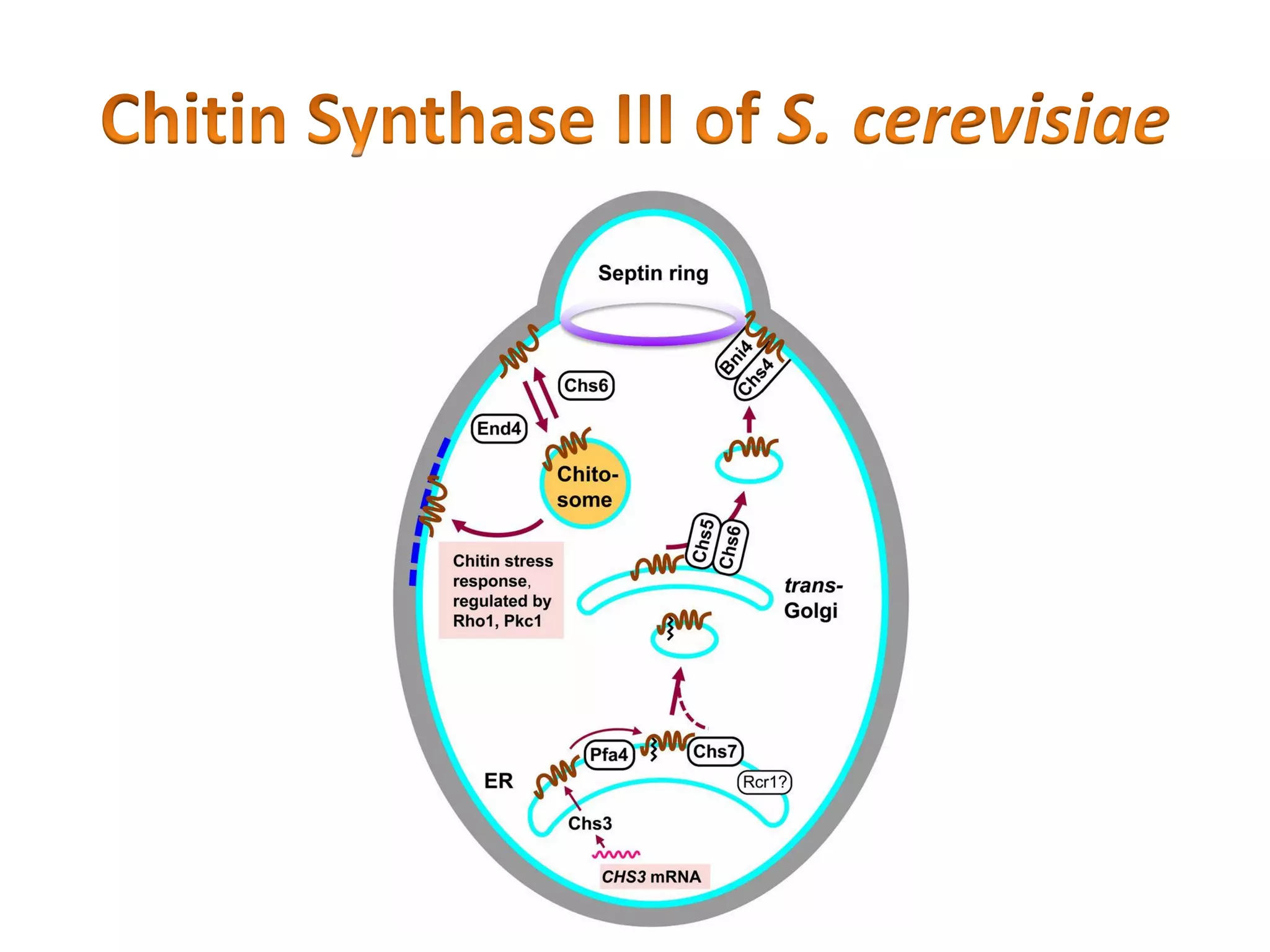
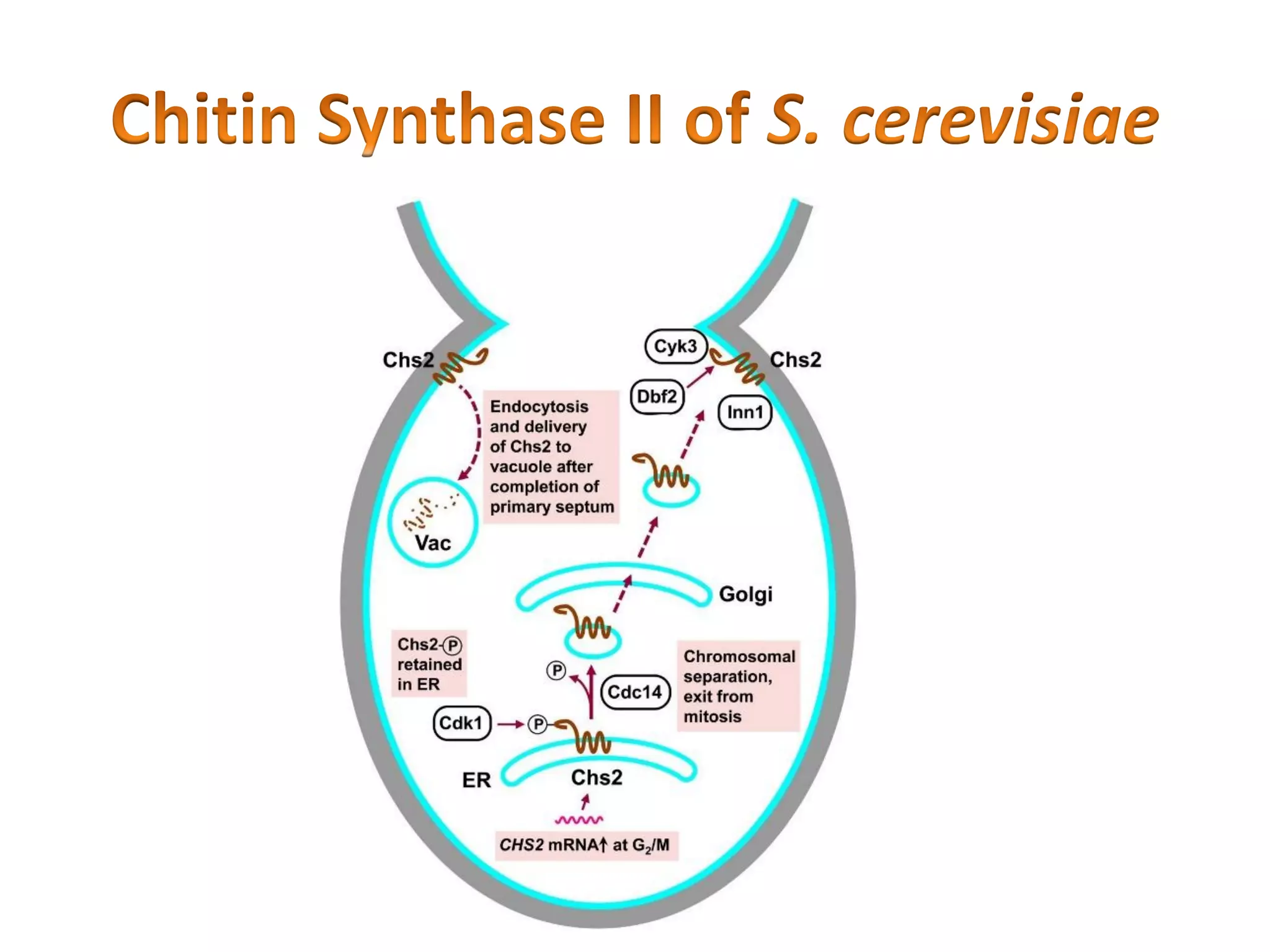
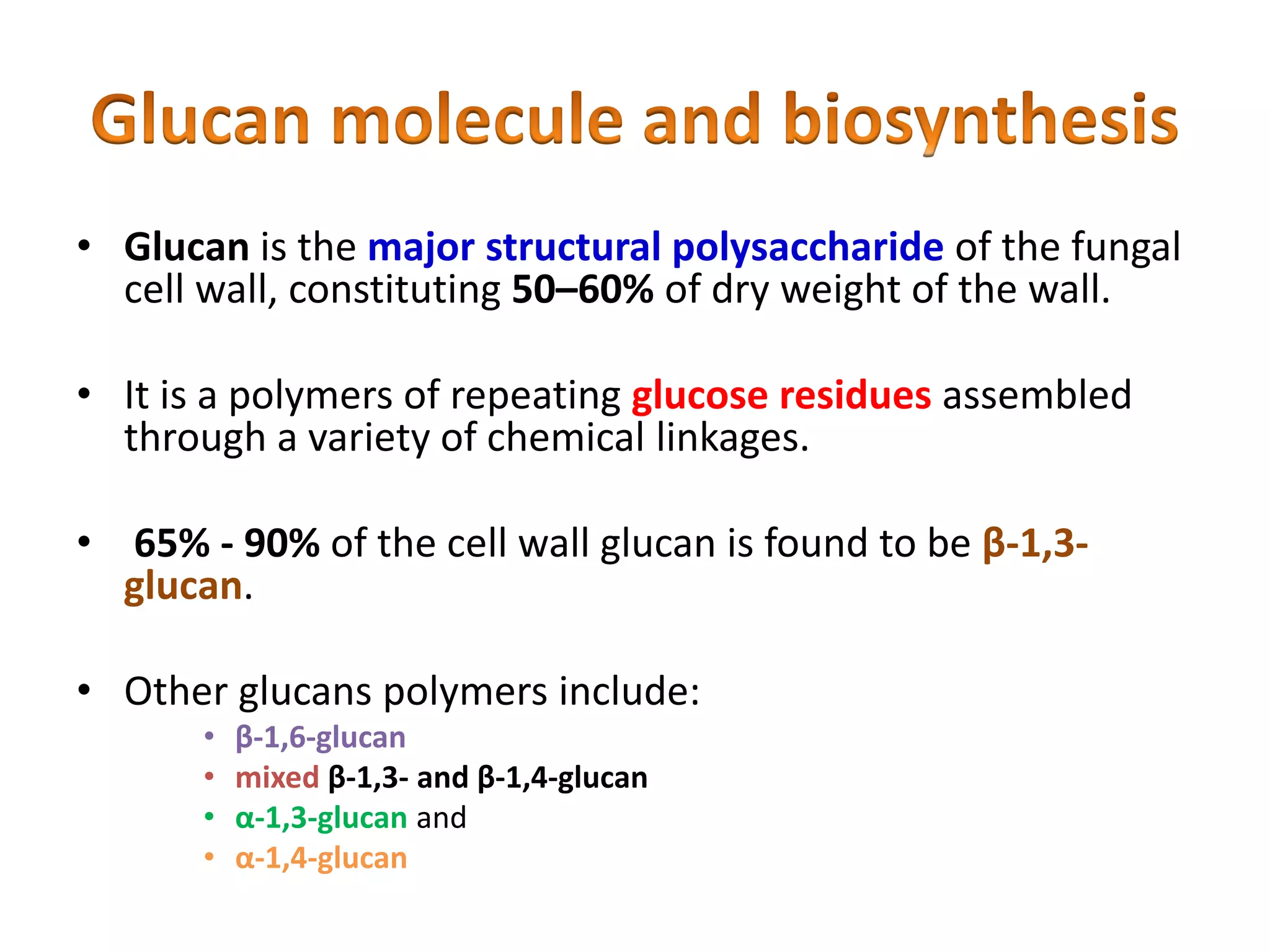
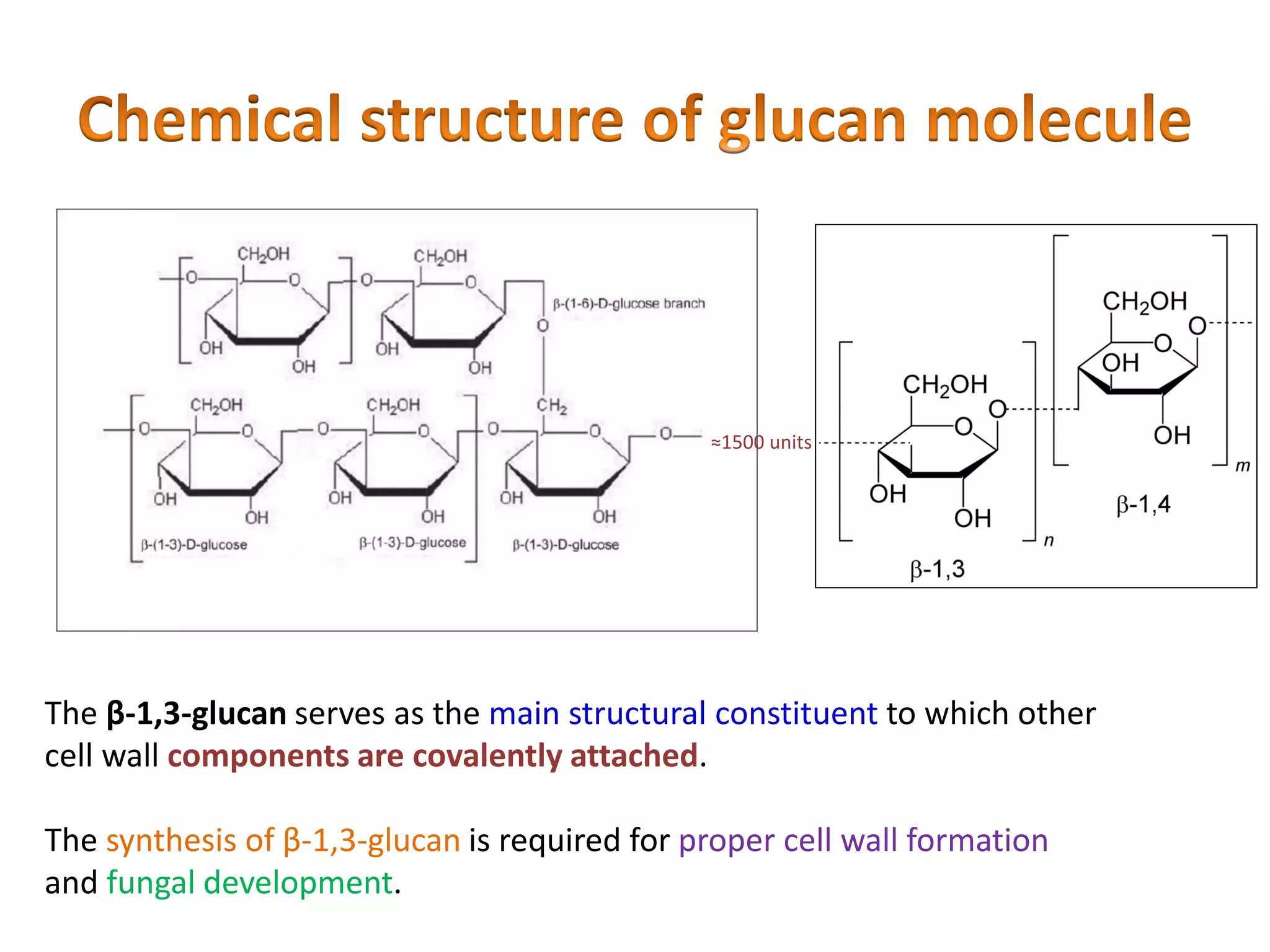
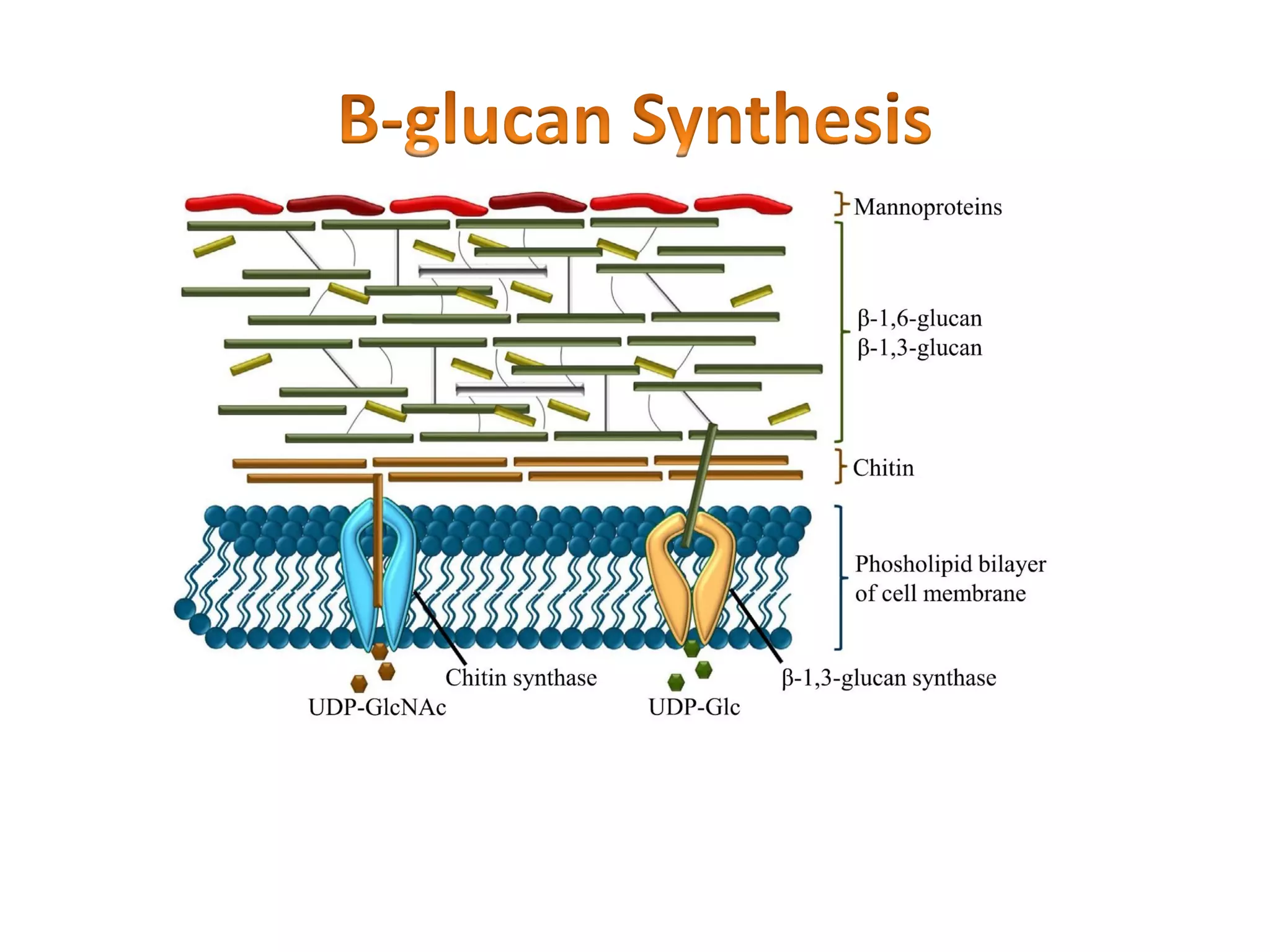
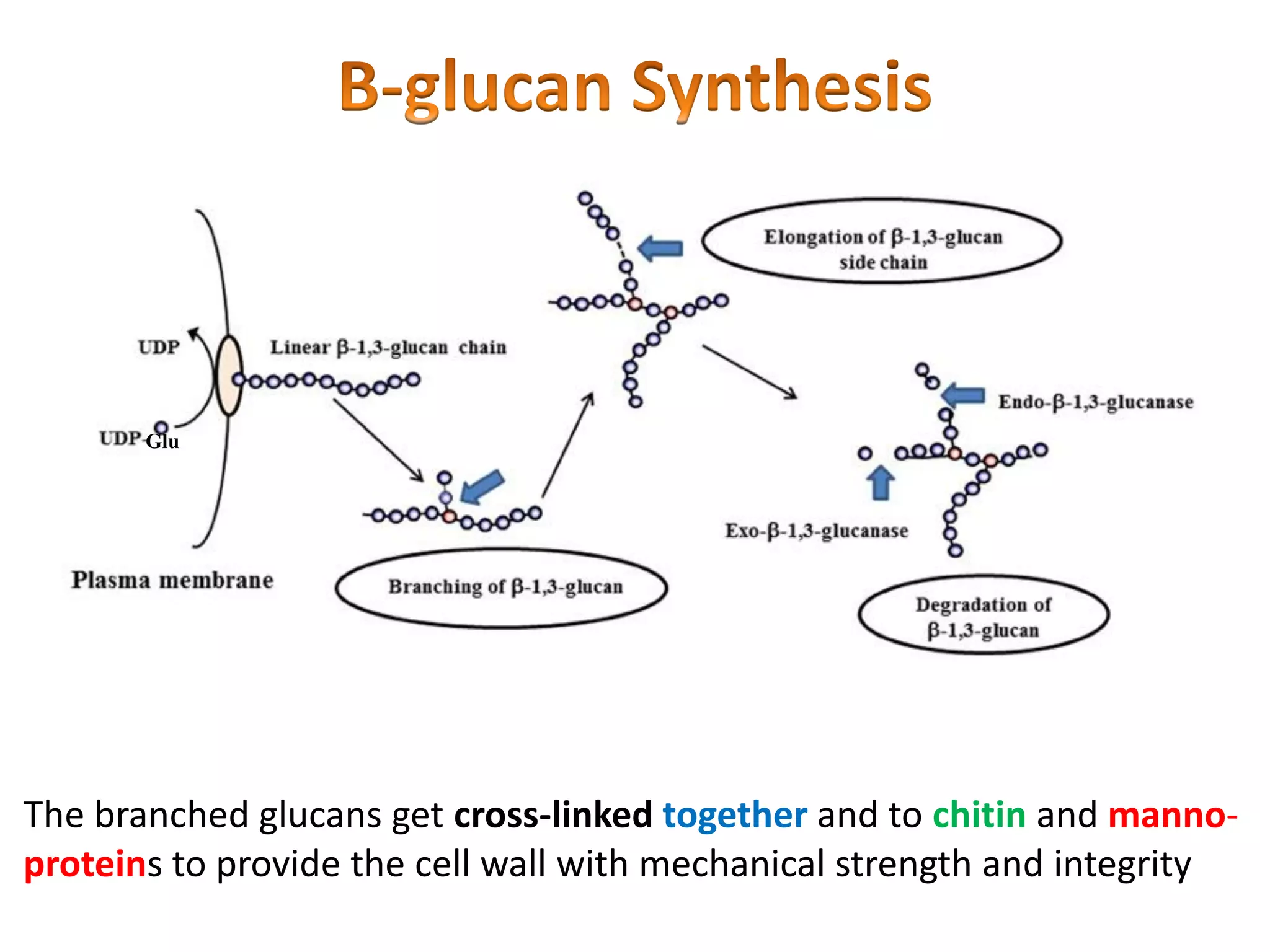
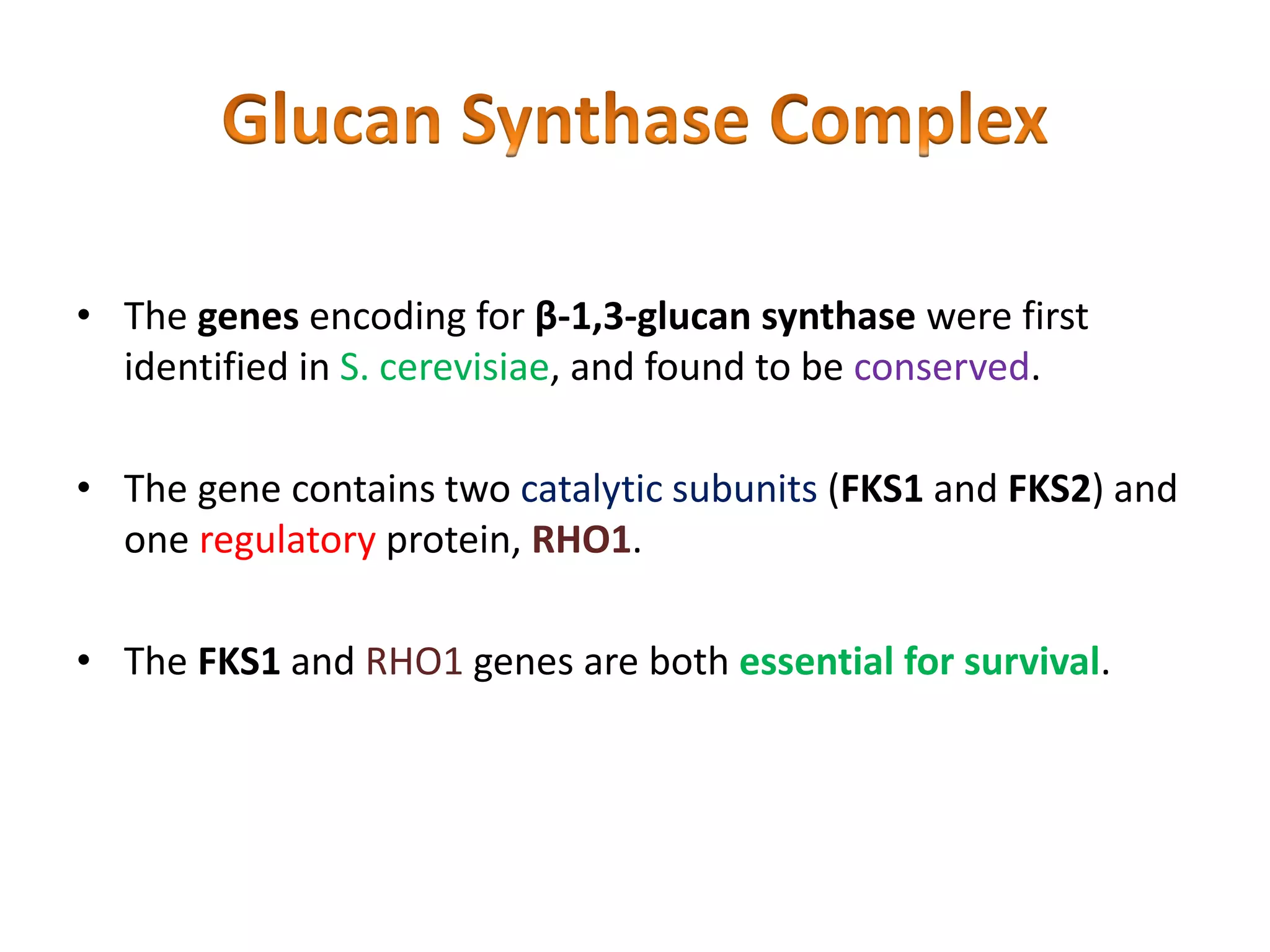
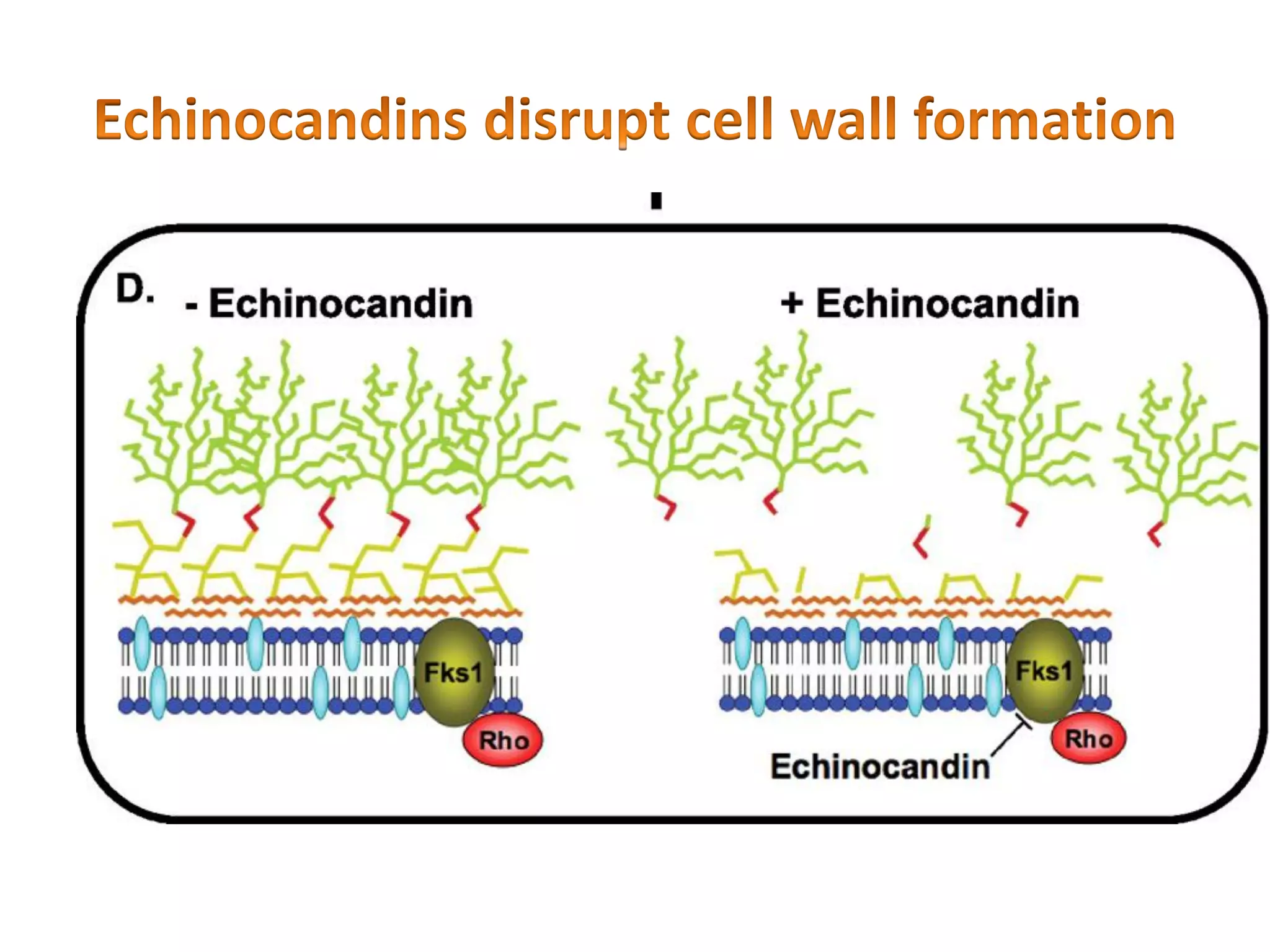
This document summarizes the structure and composition of fungal cell walls. It notes that fungal cell walls contain chitin, glucans, mannans, and glycoproteins. Chitin and glucans make up the main structural components. Glucans can be beta-1,3-glucan, beta-1,6-glucan or mixed linkages. Chitin is synthesized by chitin synthase and glucans by the glucan synthase complex. Inhibitors of these enzymes' activities are a target for antifungal drug development.