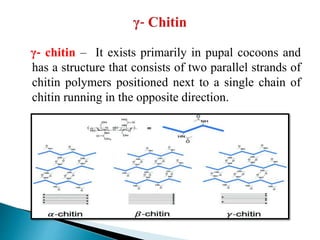
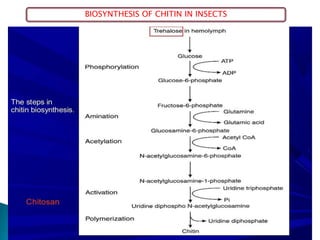
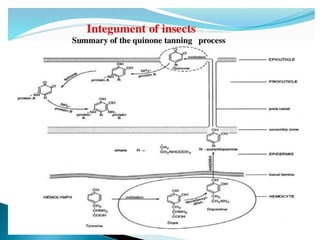
This document summarizes key information about chitin in insects. It discusses that chitin is an important biopolymer that functions as a scaffold material in insect cuticles and trachea. Insects produce chitin synthases and chitinolytic enzymes to remodel chitin structures during growth and morphogenesis. The most common form of chitin in insects is alpha-chitin, which has antiparallel polymer chains that provide mechanical strength. Chitin biosynthesis begins with trehalose and involves chitin synthase enzymes. Competitive inhibitors of chitin synthase like polyoxin D and nikkomycin Z can inhibit chitin synthesis. Sclerotization or tanning of