Embed presentation
Downloaded 34 times


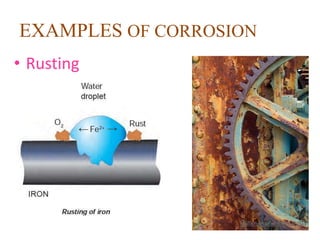



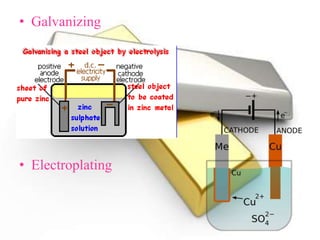
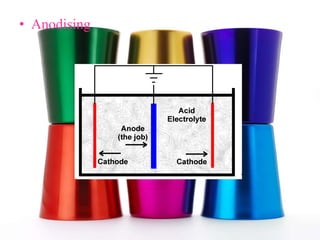




Corrosion is the gradual destruction of metals due to environmental interactions, resulting in metal compounds from chemical reactions. Examples include rusting and the corrosion of copper and silver, which can be mitigated through various protective coatings and treatments such as galvanizing and electroplating. Notably, the Iron Pillar of Delhi, over 1600 years old, has resisted rusting due to a protective oxide film.











