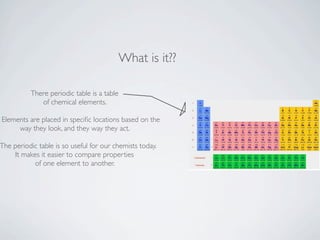
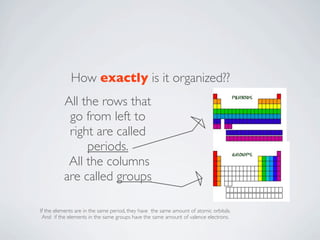
The document discusses the periodic table, which organizes the chemical elements based on their atomic structure and properties. It explains that Dmitri Mendeleev created the periodic table by arranging the known elements in order of atomic weight and leaving gaps for elements that had not yet been discovered. Examples are given of three elements - cerium, oxygen, and hydrogen - including their atomic properties and common uses.