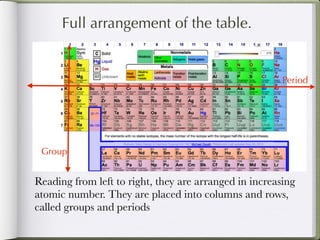
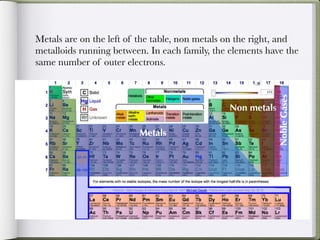
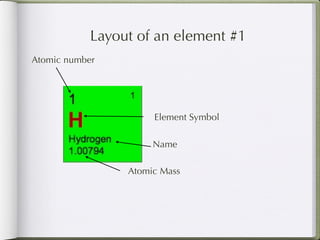
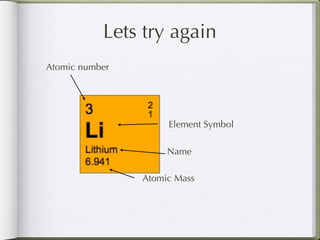
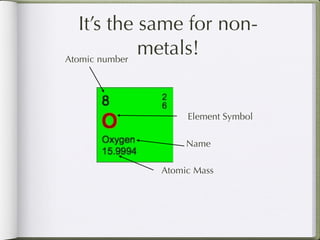
Dmitri Mendeleeve, a Russian chemist, is credited with inventing the Periodic Table in the late 1800s. He arranged the known elements by their atomic mass to find a pattern, placing them into columns and rows. This allowed elements to be organized based on their atomic number and properties like metals being on the left, nonmetals on the right, and metalloids in between. The document then provides an example of how each element is consistently displayed with its atomic number, symbol, name, and atomic mass.