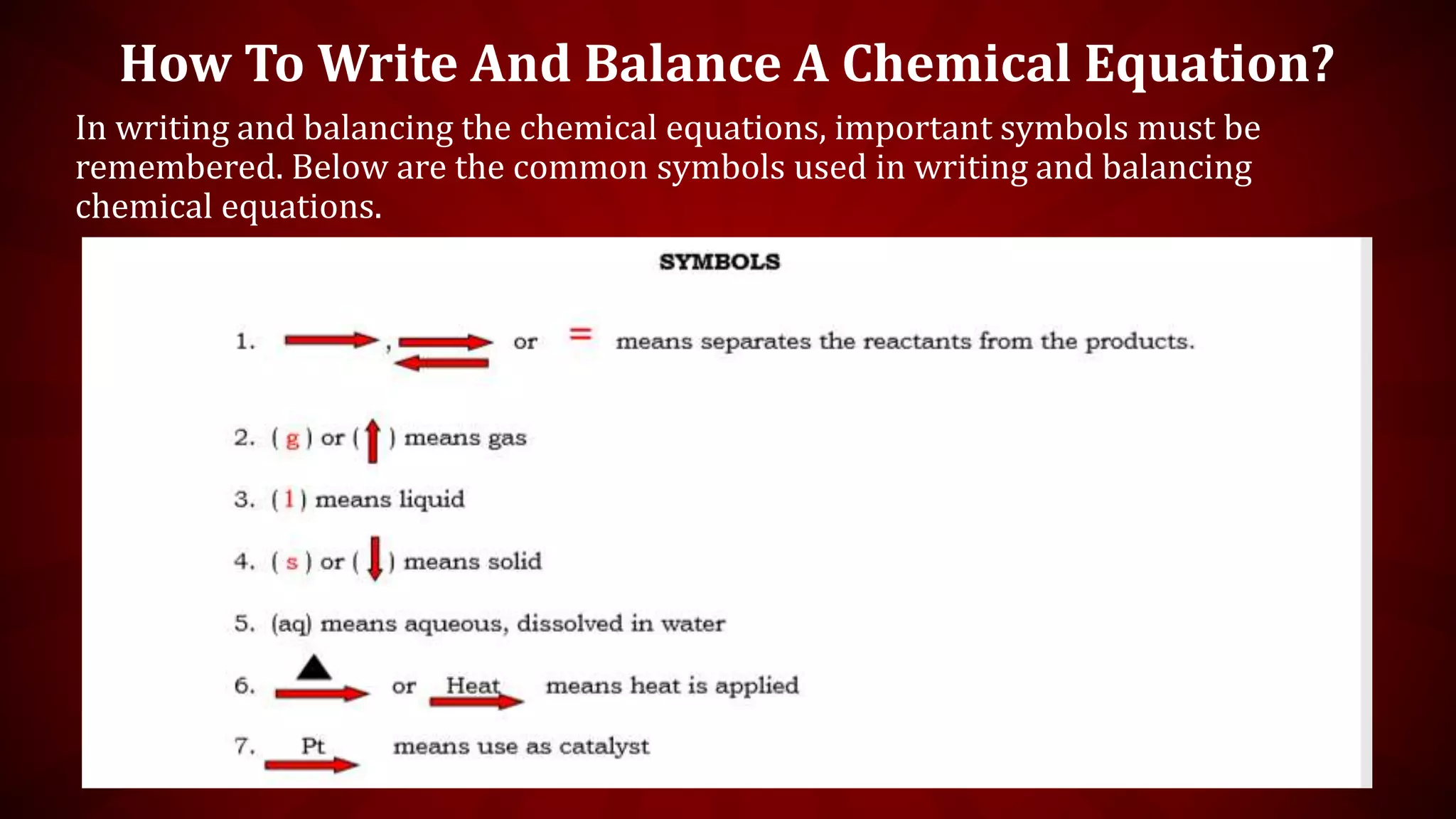
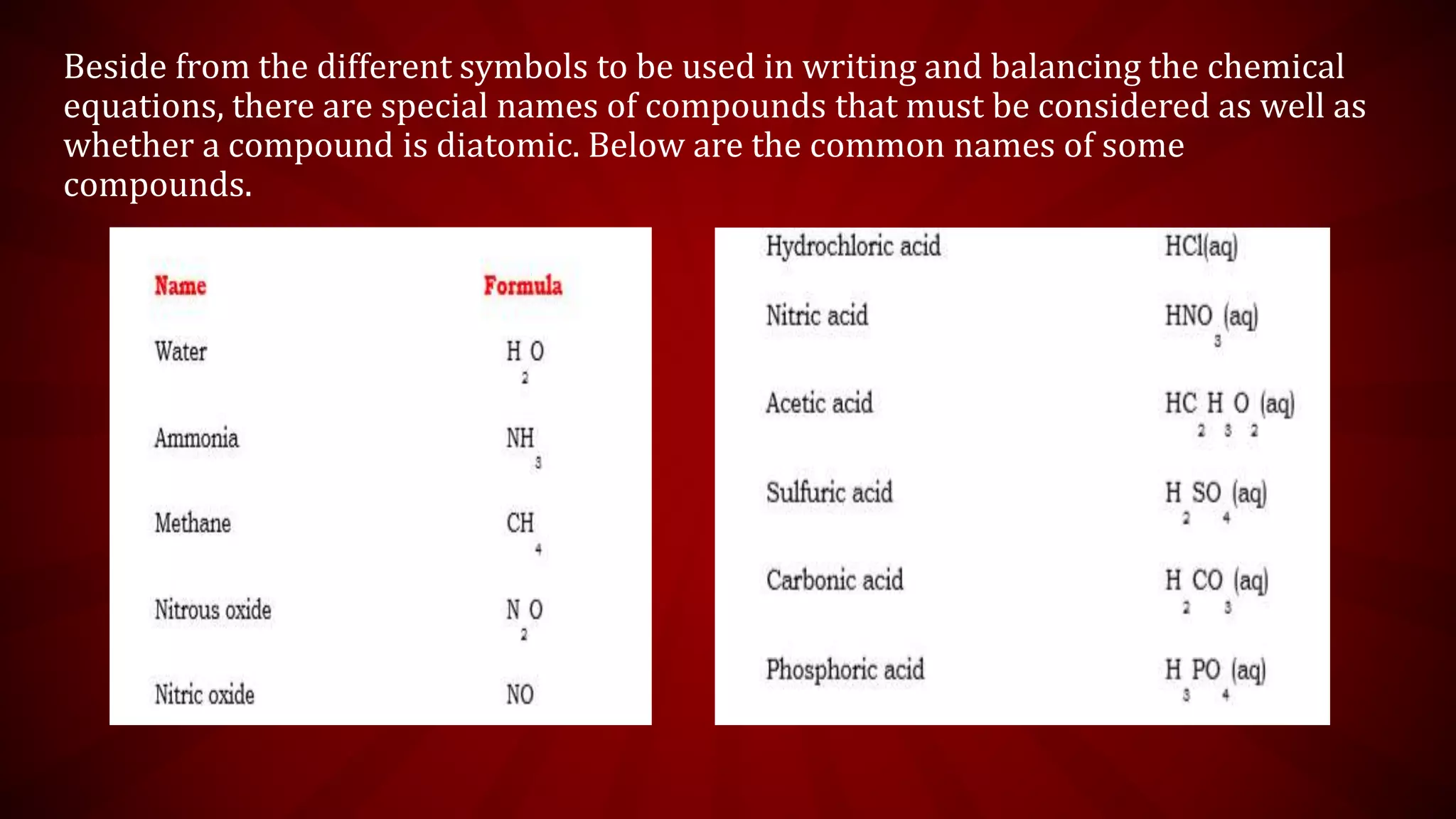
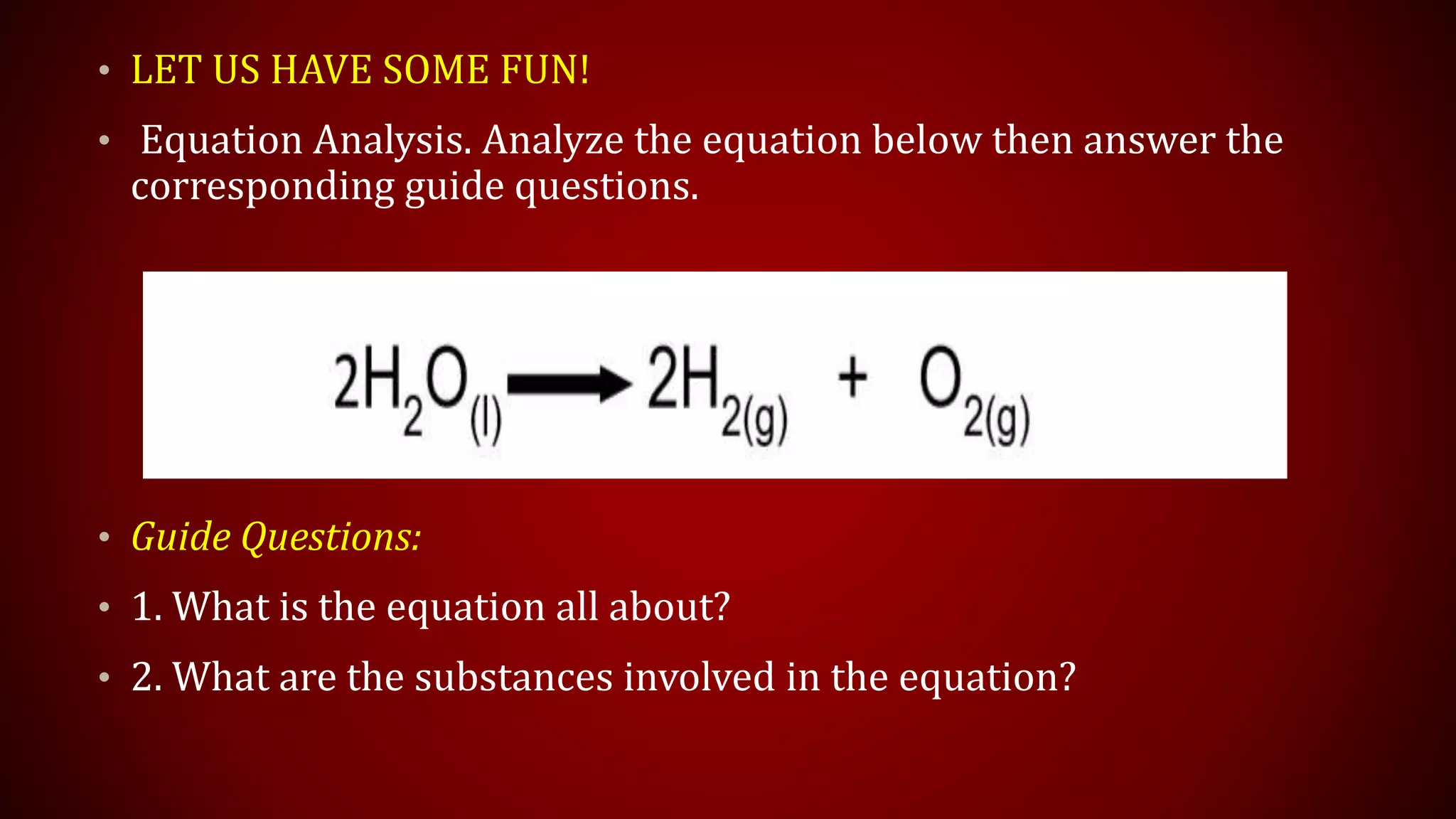
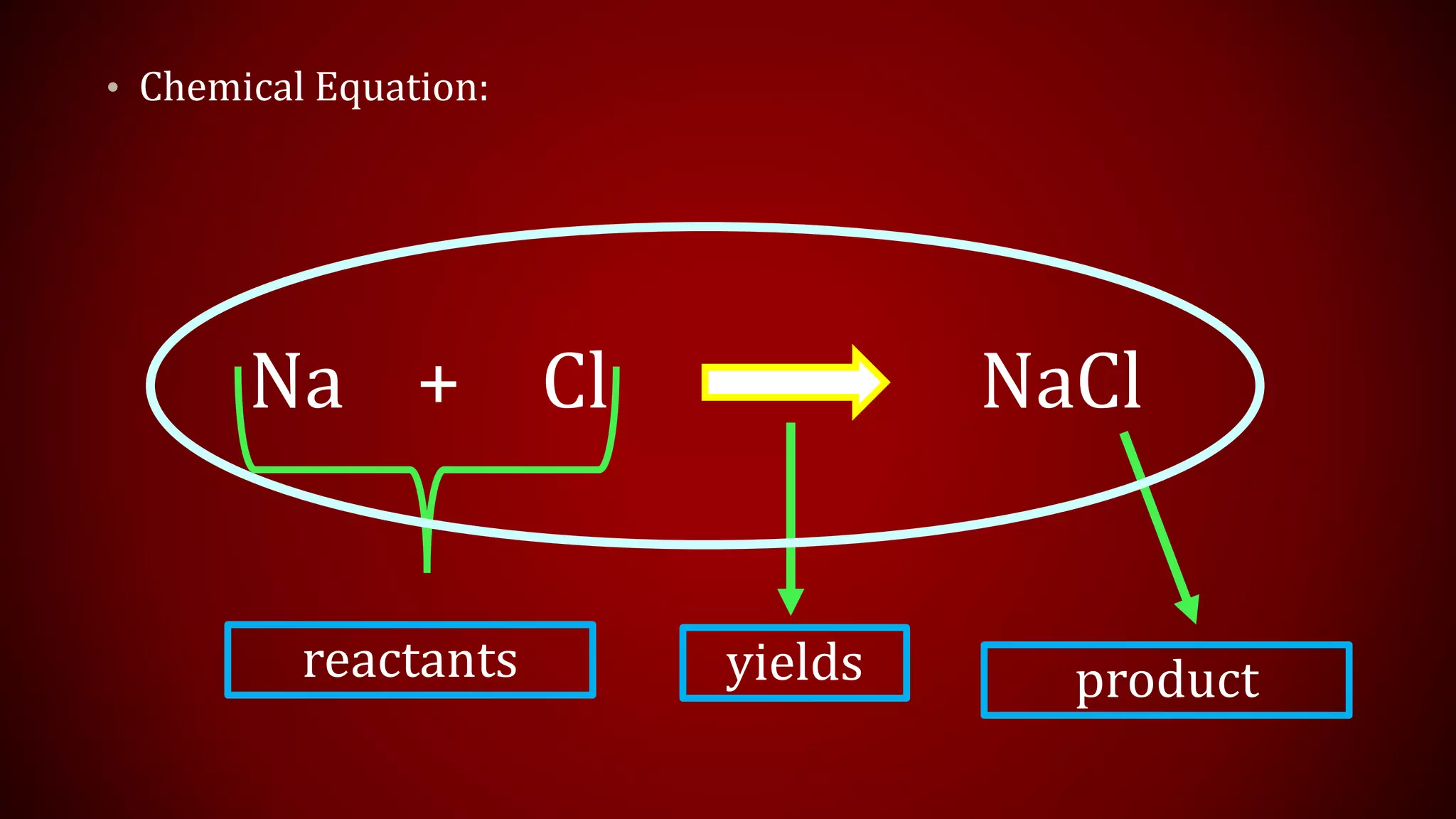
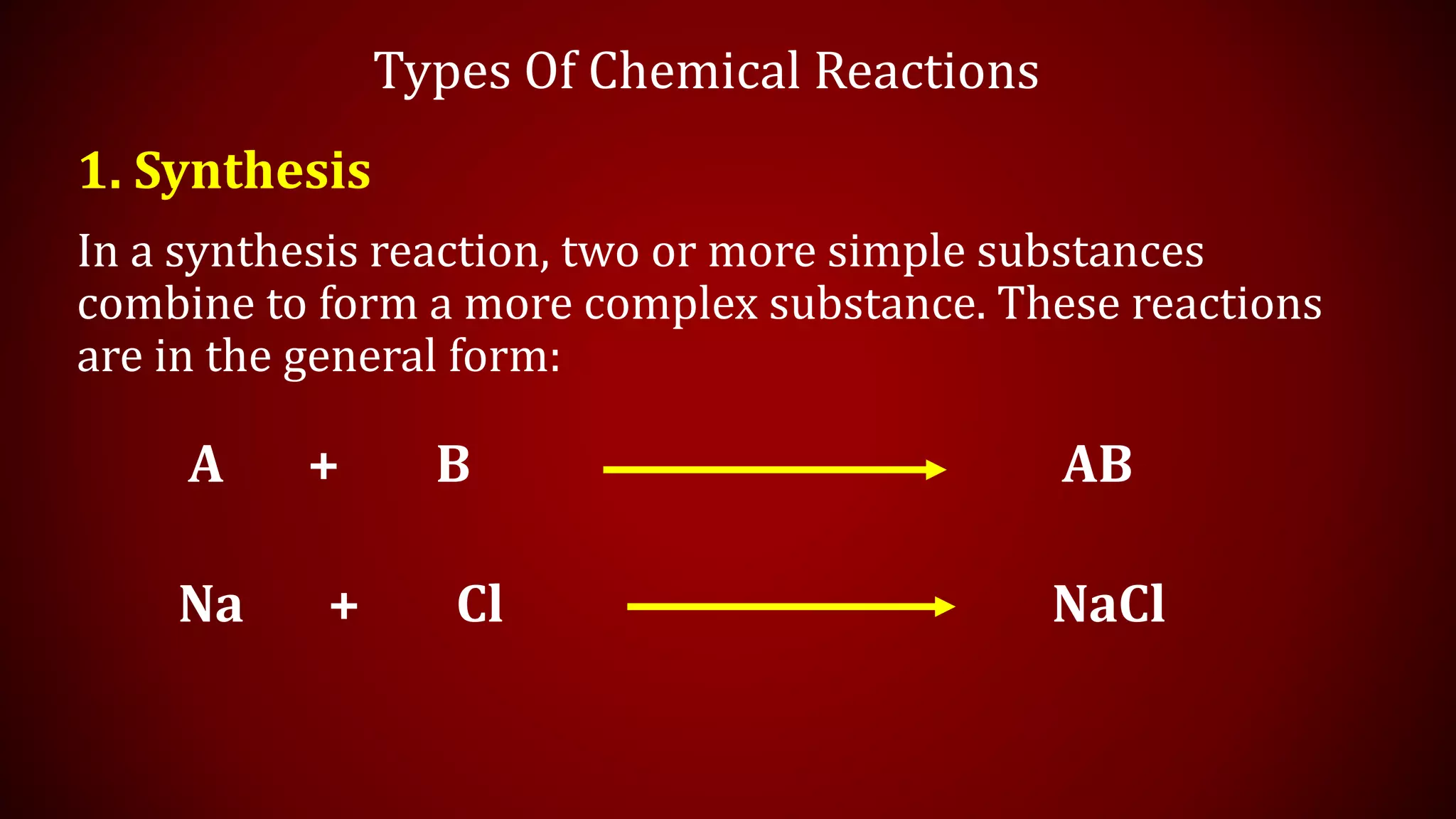
This document discusses chemical equations and types of chemical reactions. It defines chemical reactions and equations, and explains the key parts of a chemical equation. The main types of chemical reactions covered are synthesis, decomposition, single replacement, and double replacement reactions. Steps for writing and balancing chemical equations are also provided, including an example showing the process.





![Types Of Chemical Reactions
4. Double replacement
In a double replacement reaction, the anions and cations of
two compounds switch places and form two entirely
different compounds.[18] These reactions are in the general
form
AB + CD AD + CB
Pb(NO ) + 2KI PbI + 2KNO
3 2 3
2](https://image.slidesharecdn.com/chemicalequations-221012154822-00a1b64f/75/chemical-EQUATIONS-pptx-9-2048.jpg)