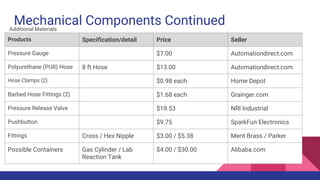
The document describes a spring pressure stopping mechanism for a ChemE Car. A chemical reaction produces gas pressure that pushes a piston against a spring. When the pressure overcomes the spring force, the piston hits a switch to stop the car's propulsion. Sulfuric acid and calcium carbonate, and other reactions, were considered. Components like cylinders, springs, gauges and their costs are outlined. Safety procedures, regulations and potential issues are also discussed.