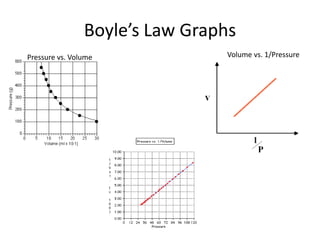
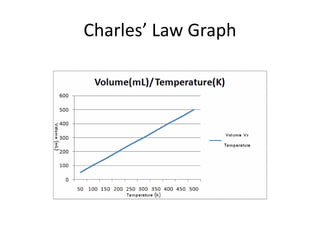
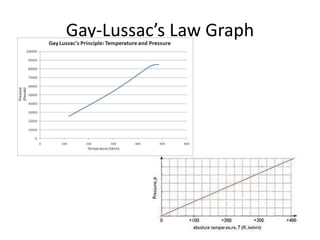
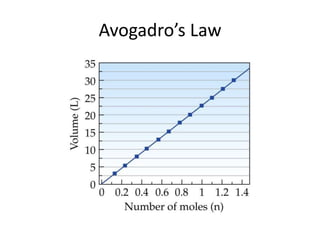
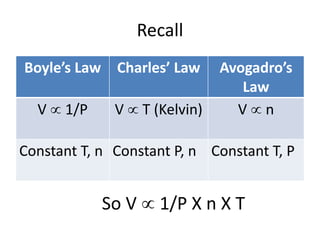
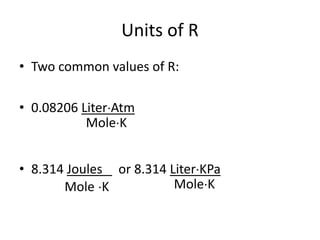
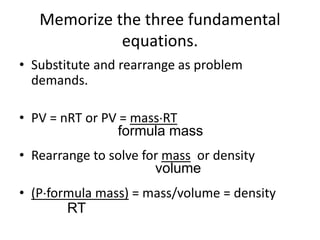
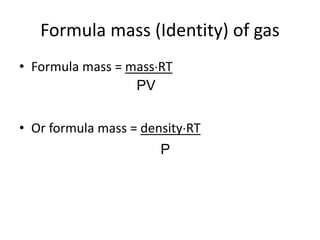
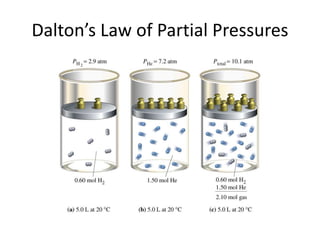
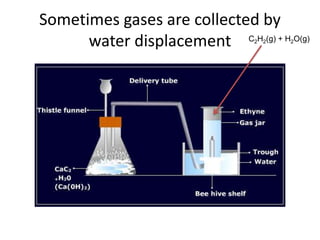
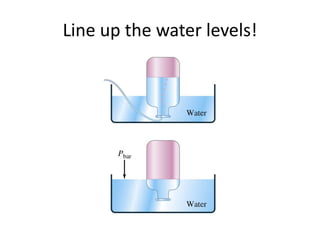
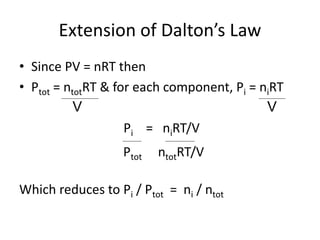
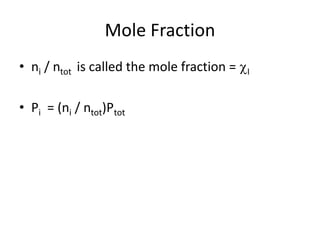
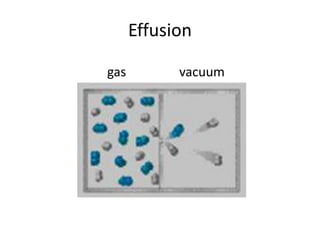
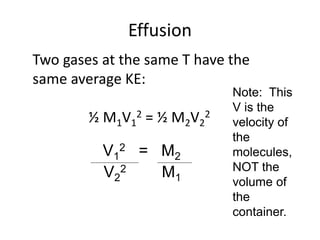
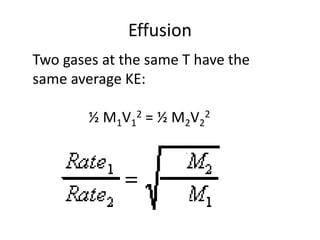
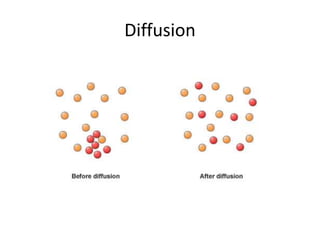
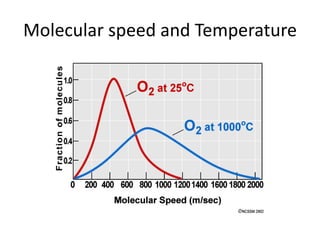
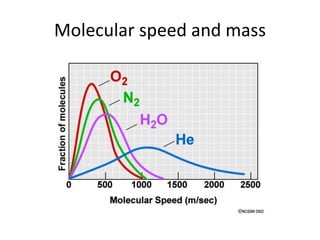
The document discusses the ideal gas law and its applications. It describes the four variables that characterize a gas sample - temperature, pressure, volume, and amount of gas. The ideal gas law equation relates these variables and can be used to determine one variable if the other three are known. The document also discusses effusion and diffusion, describing how the rates of these processes depend on molecular mass and temperature based on the kinetic energy of gas particles.