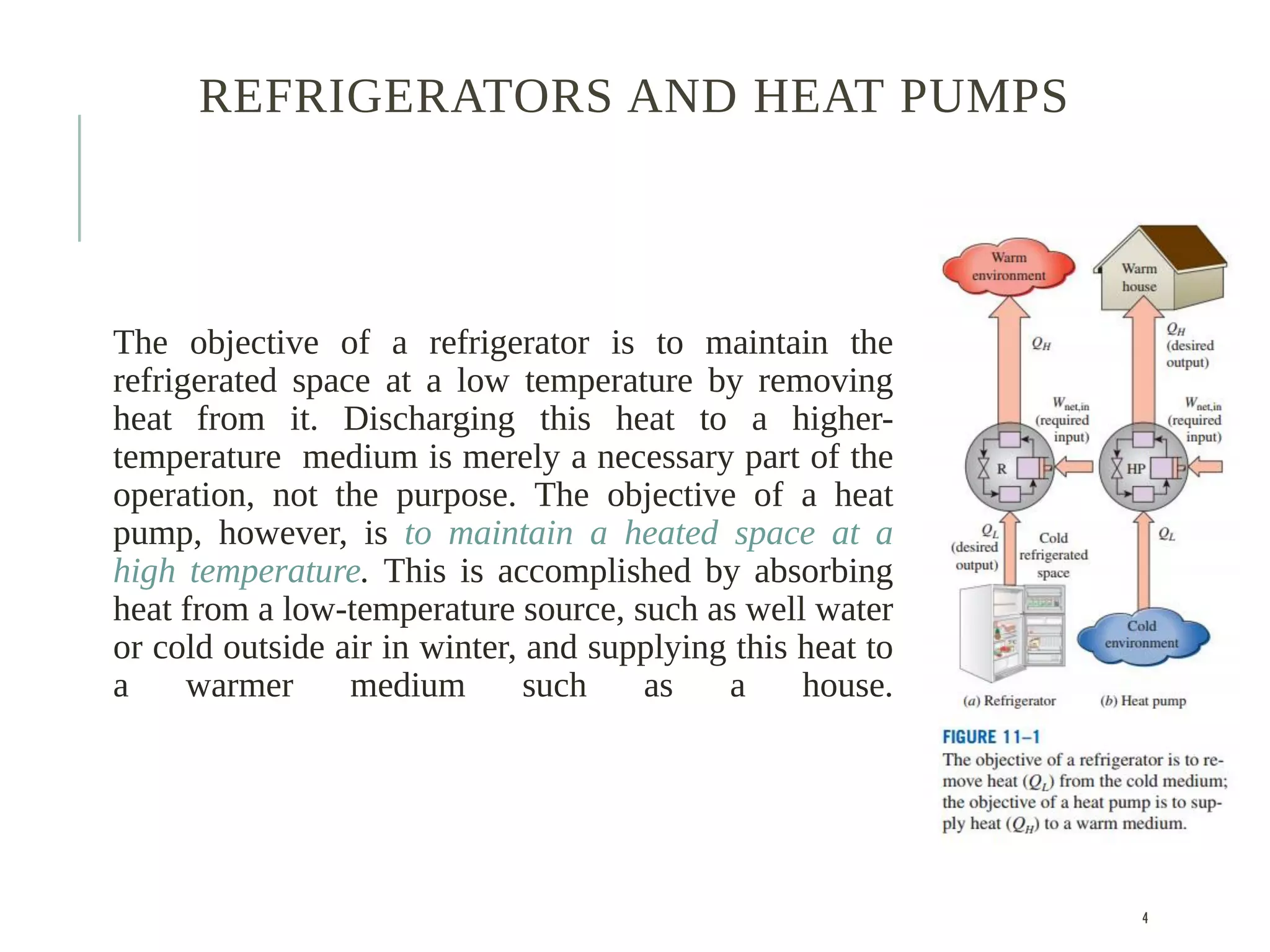
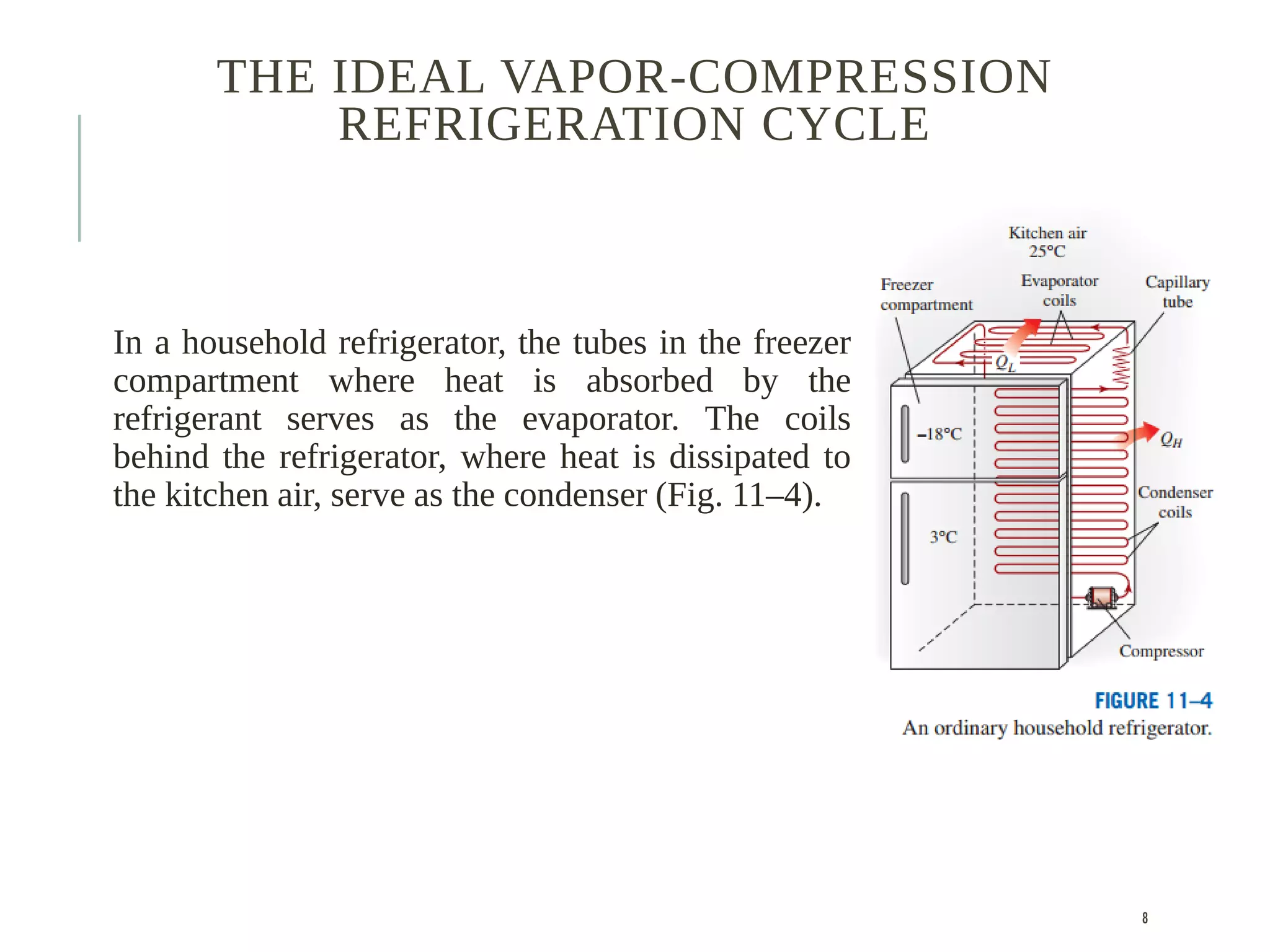
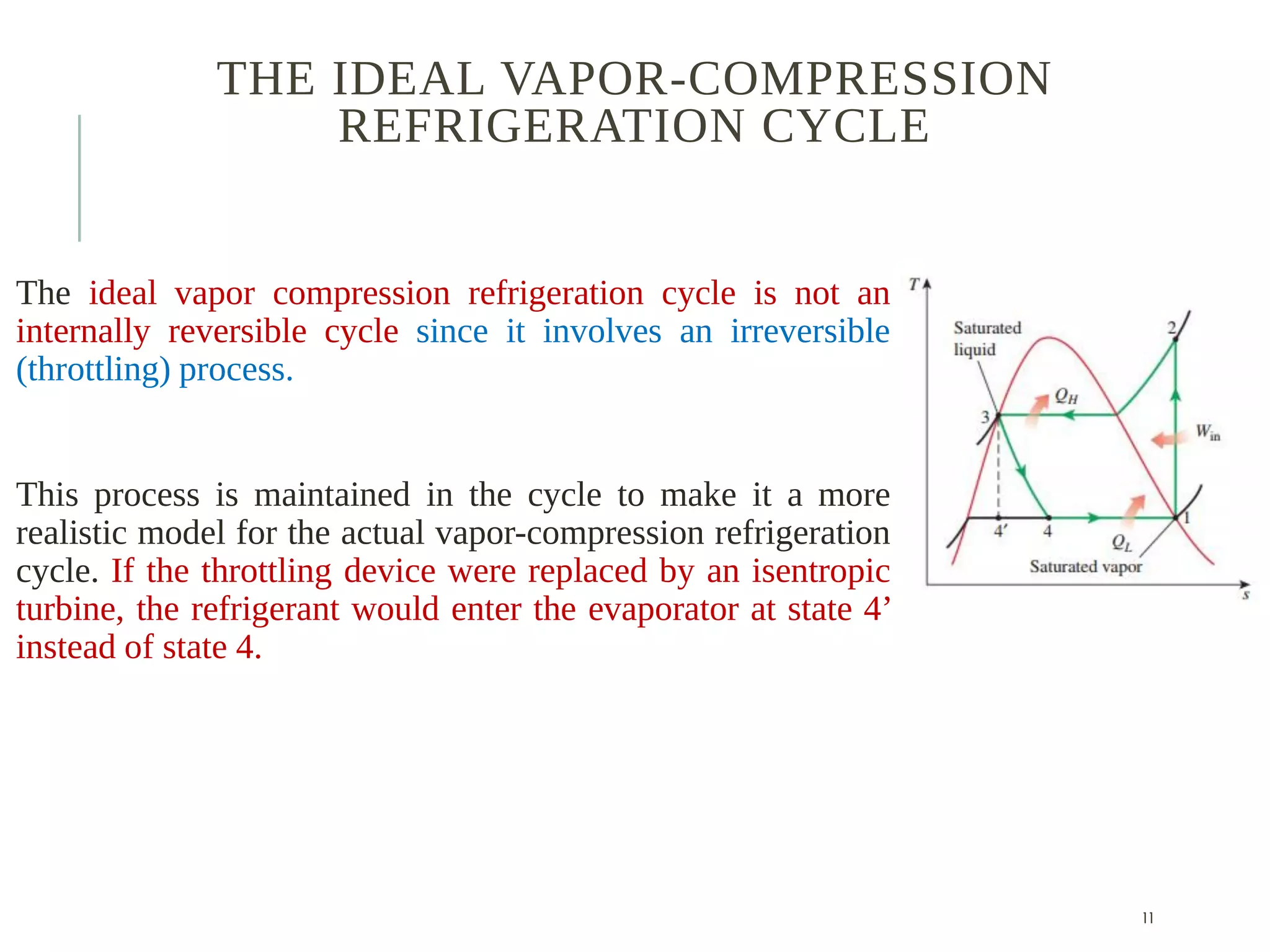
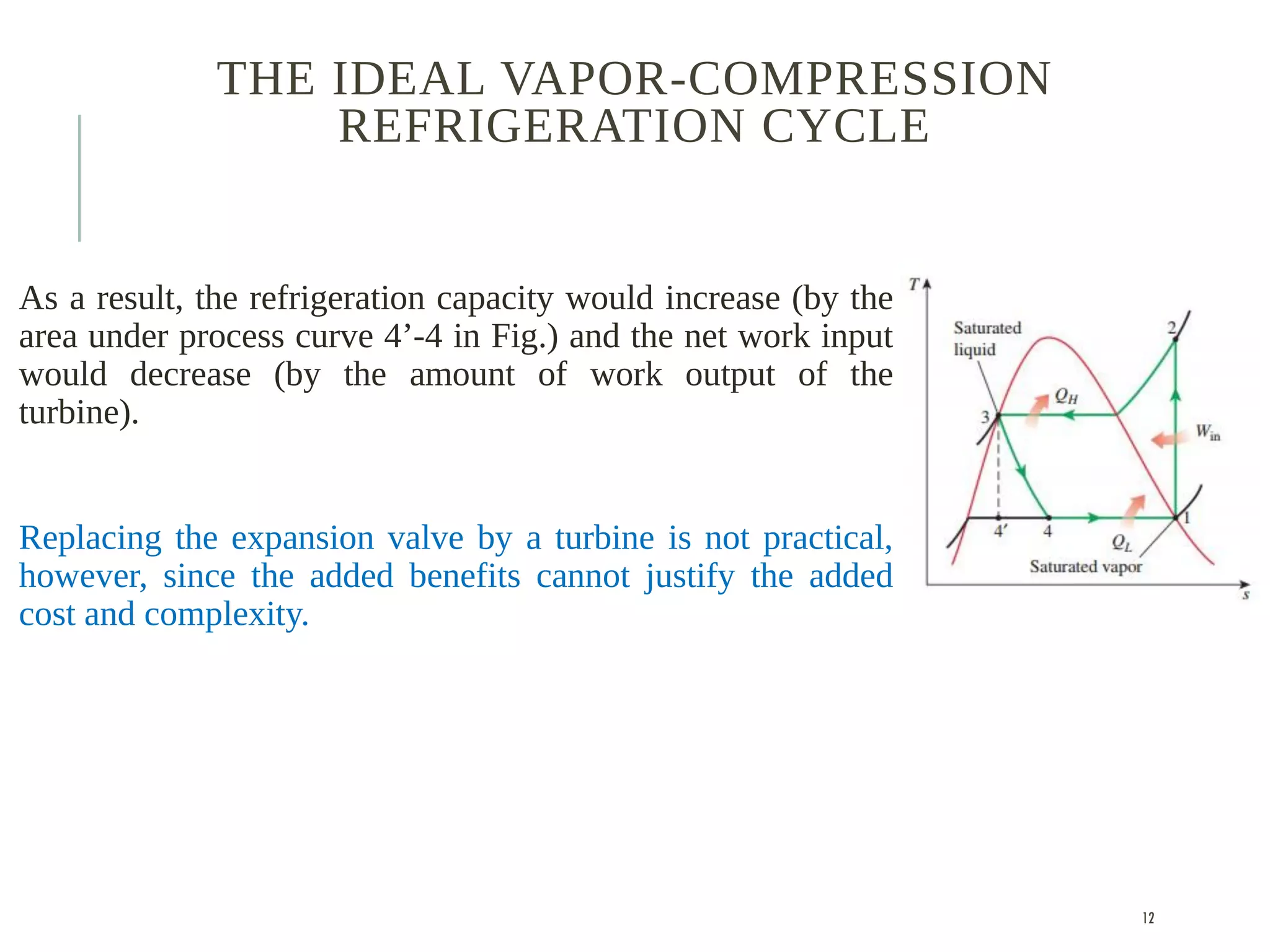
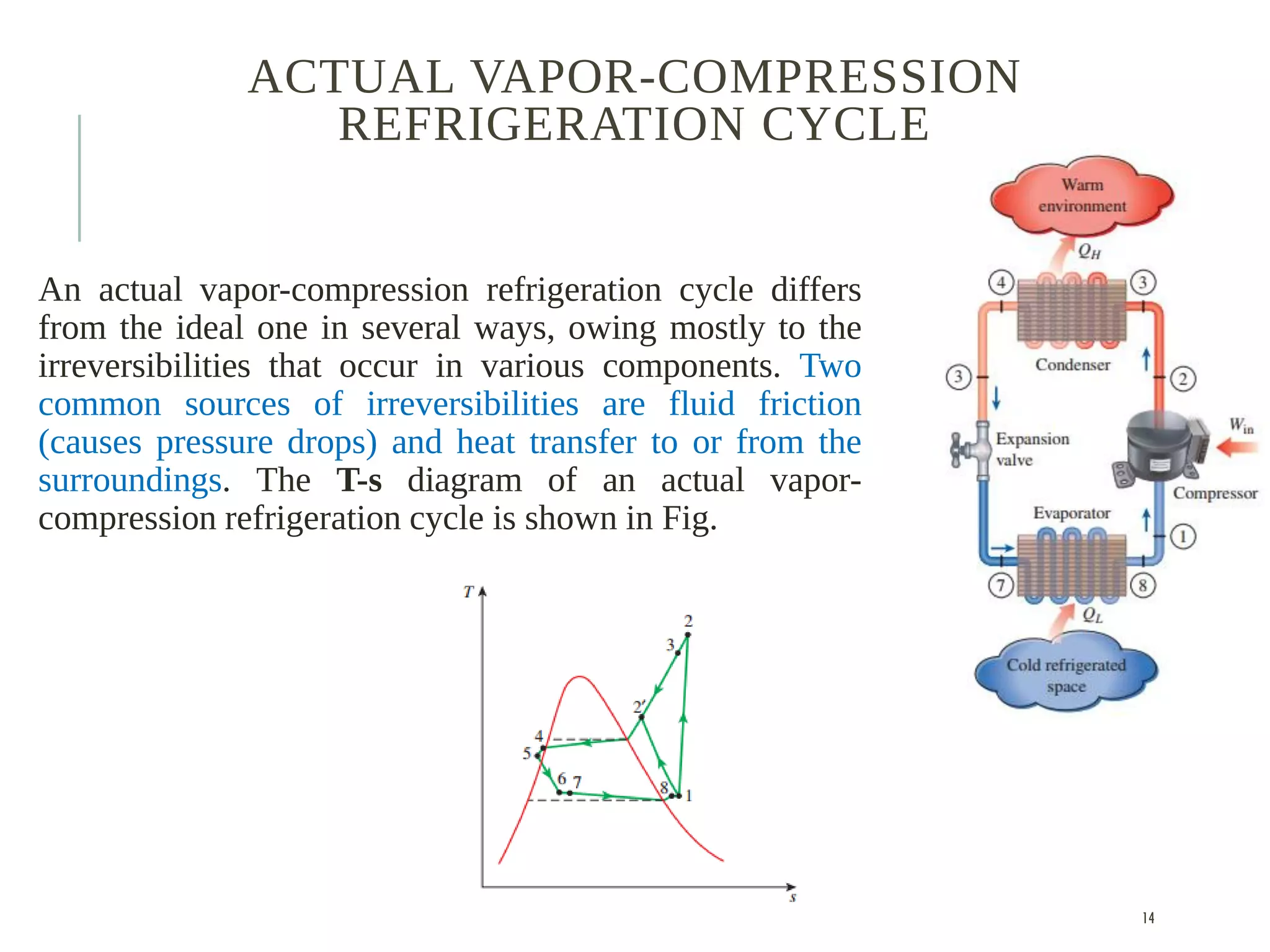
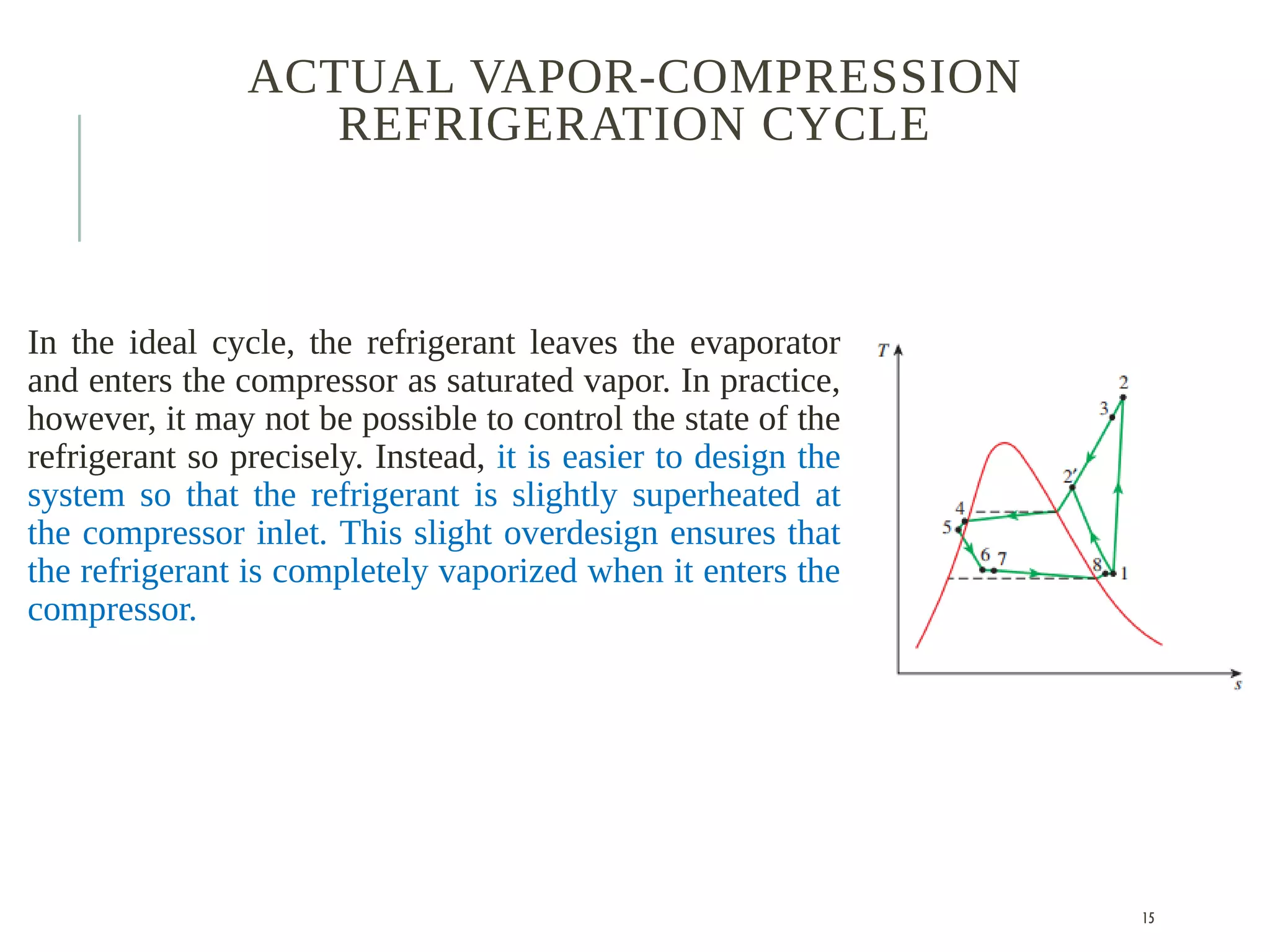
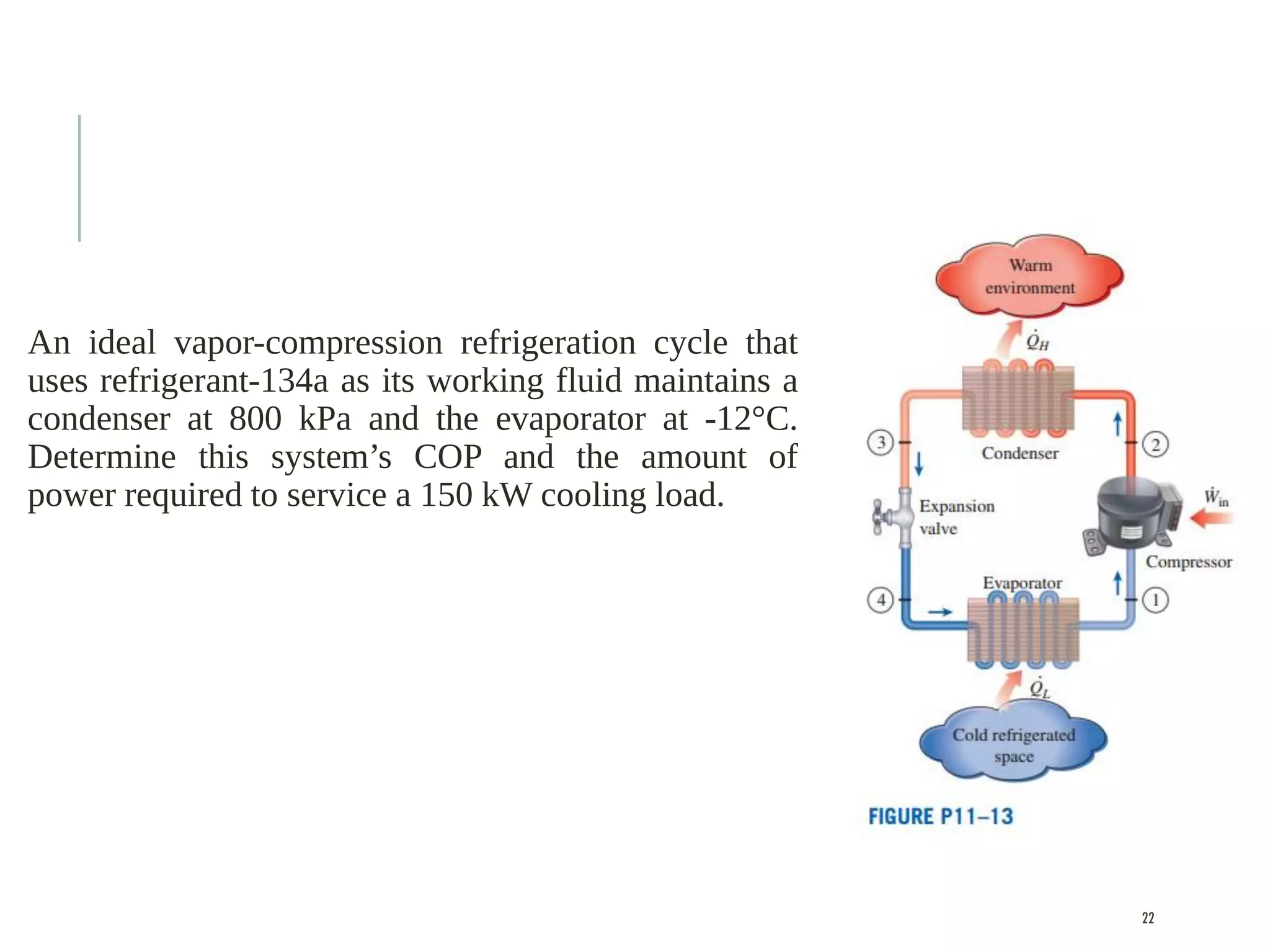
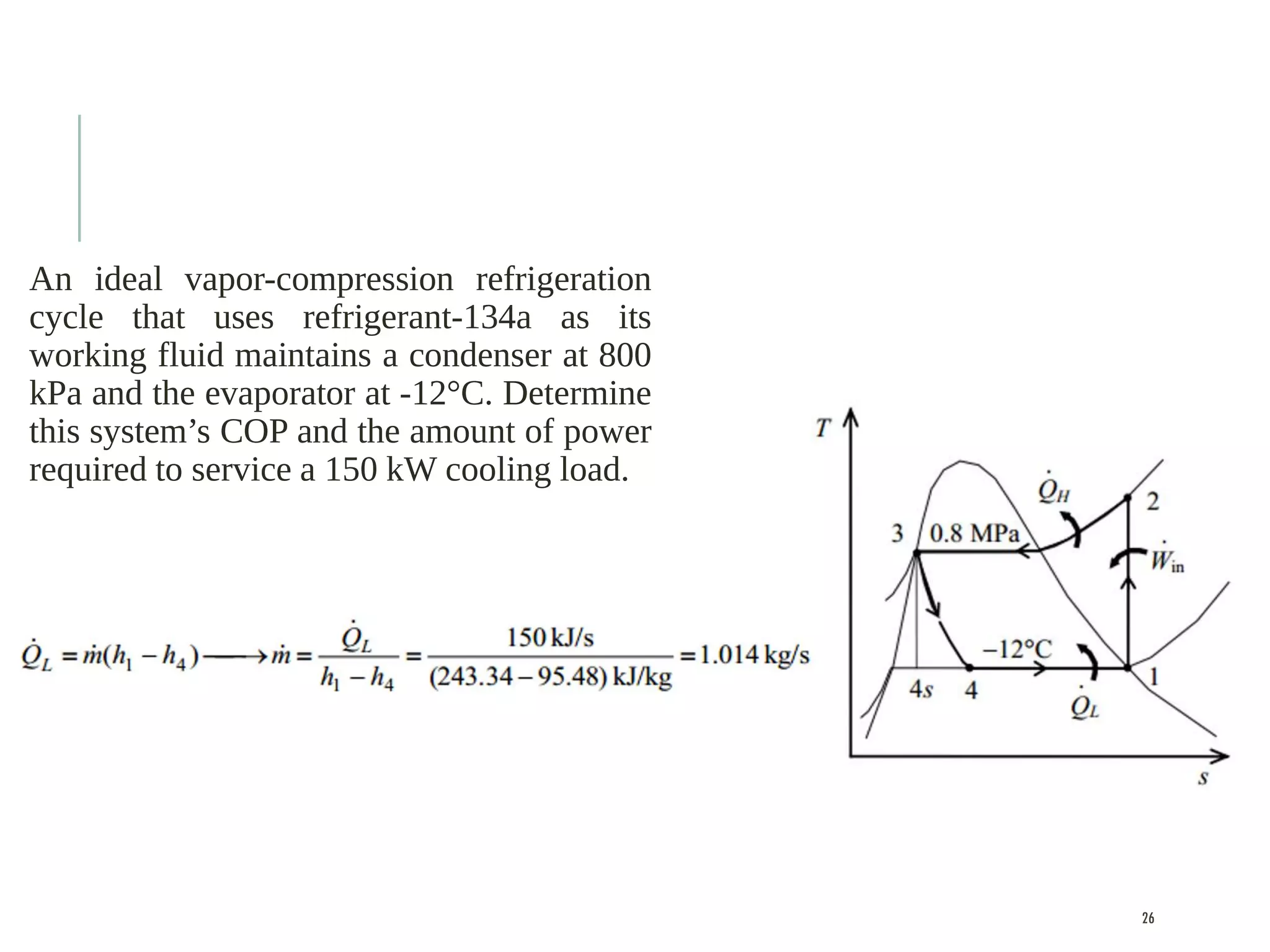
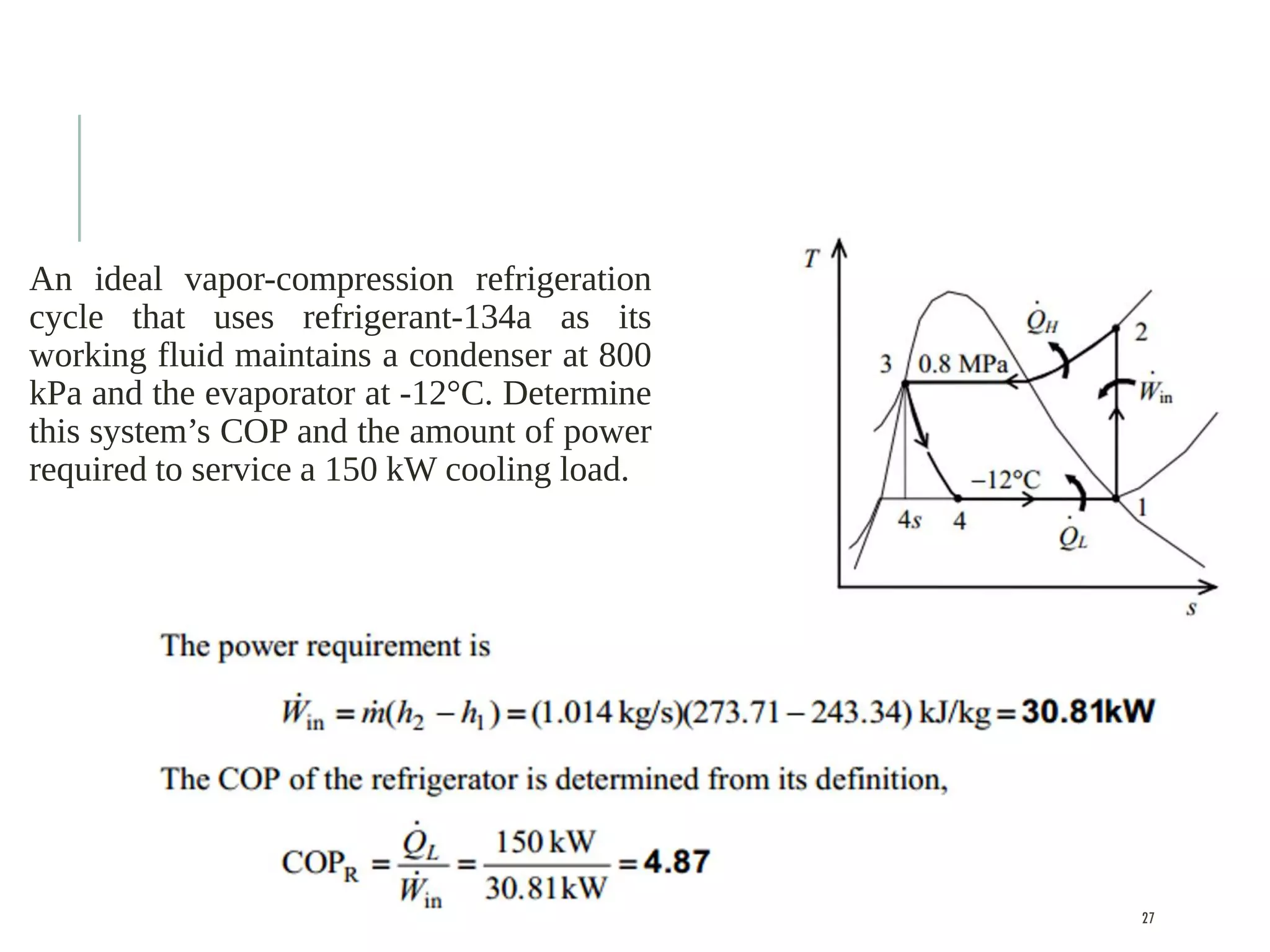
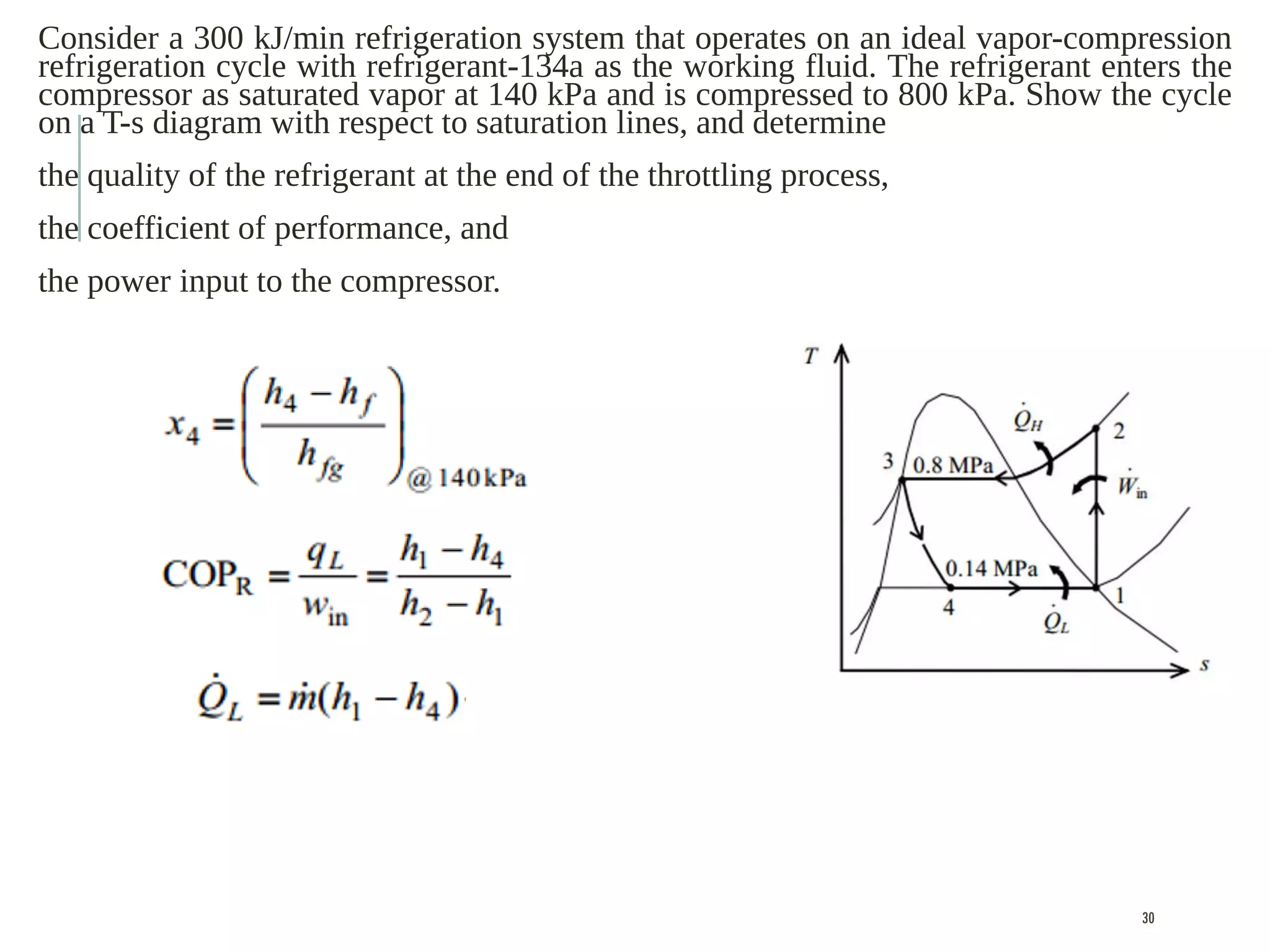
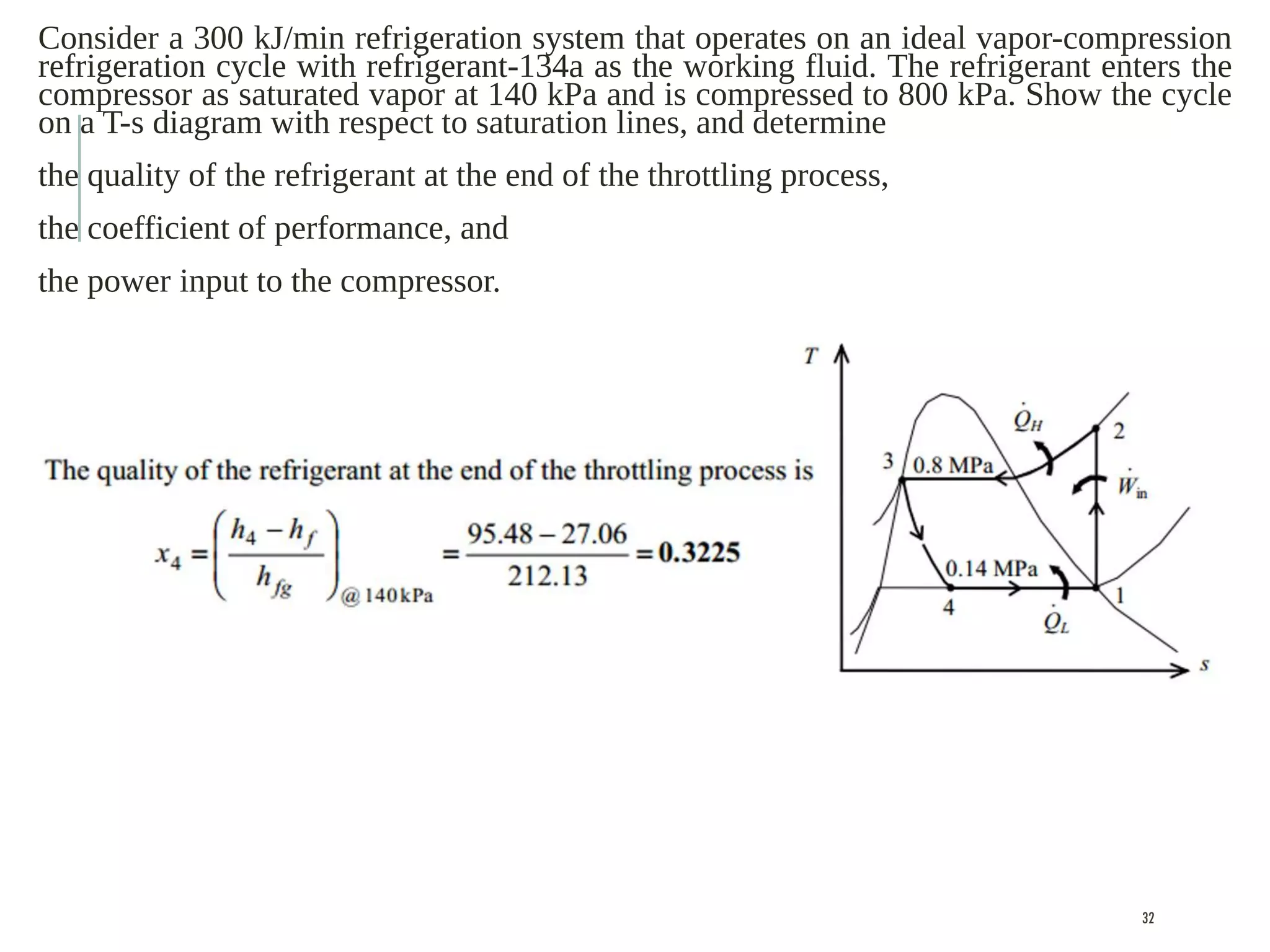
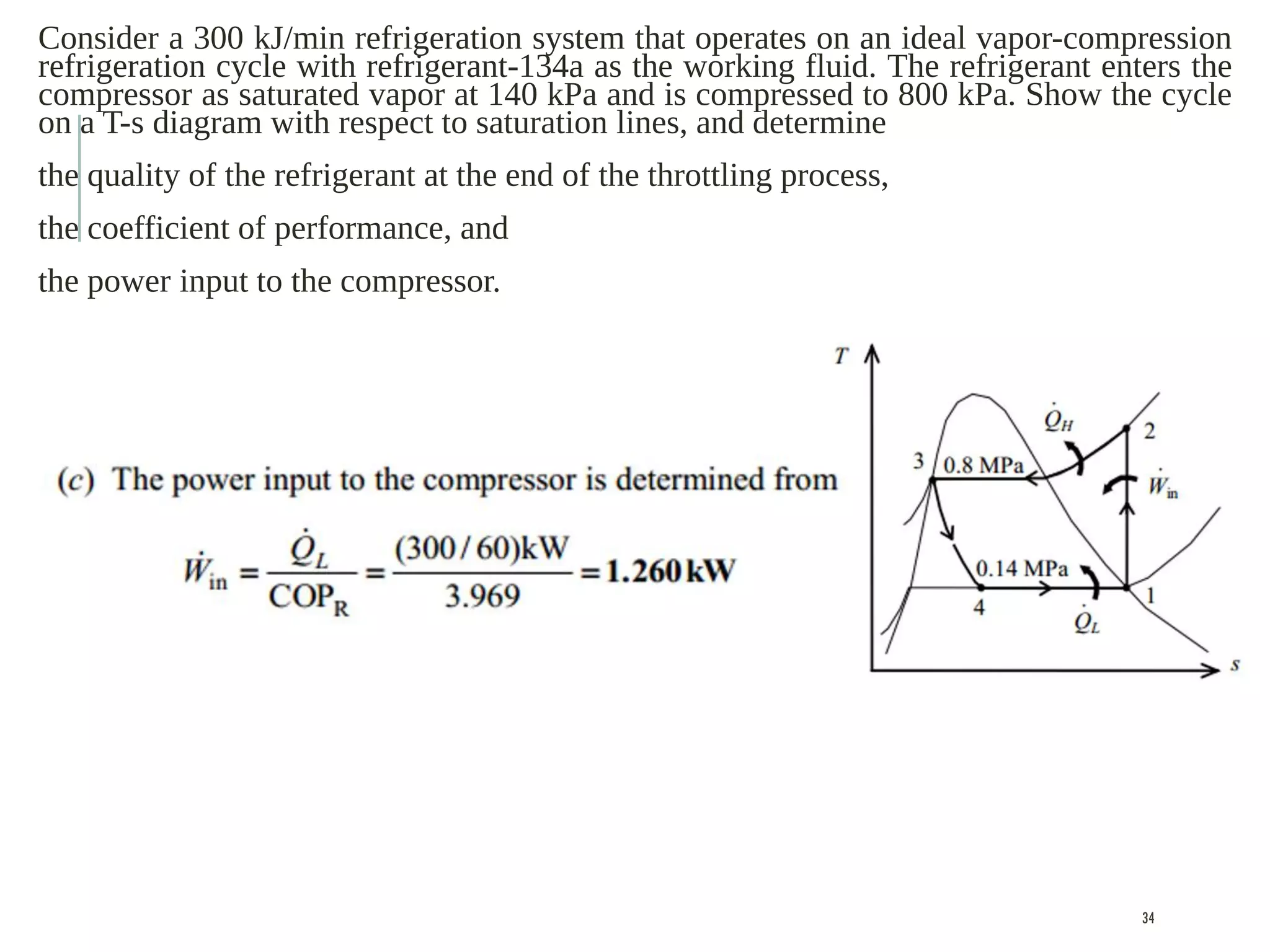
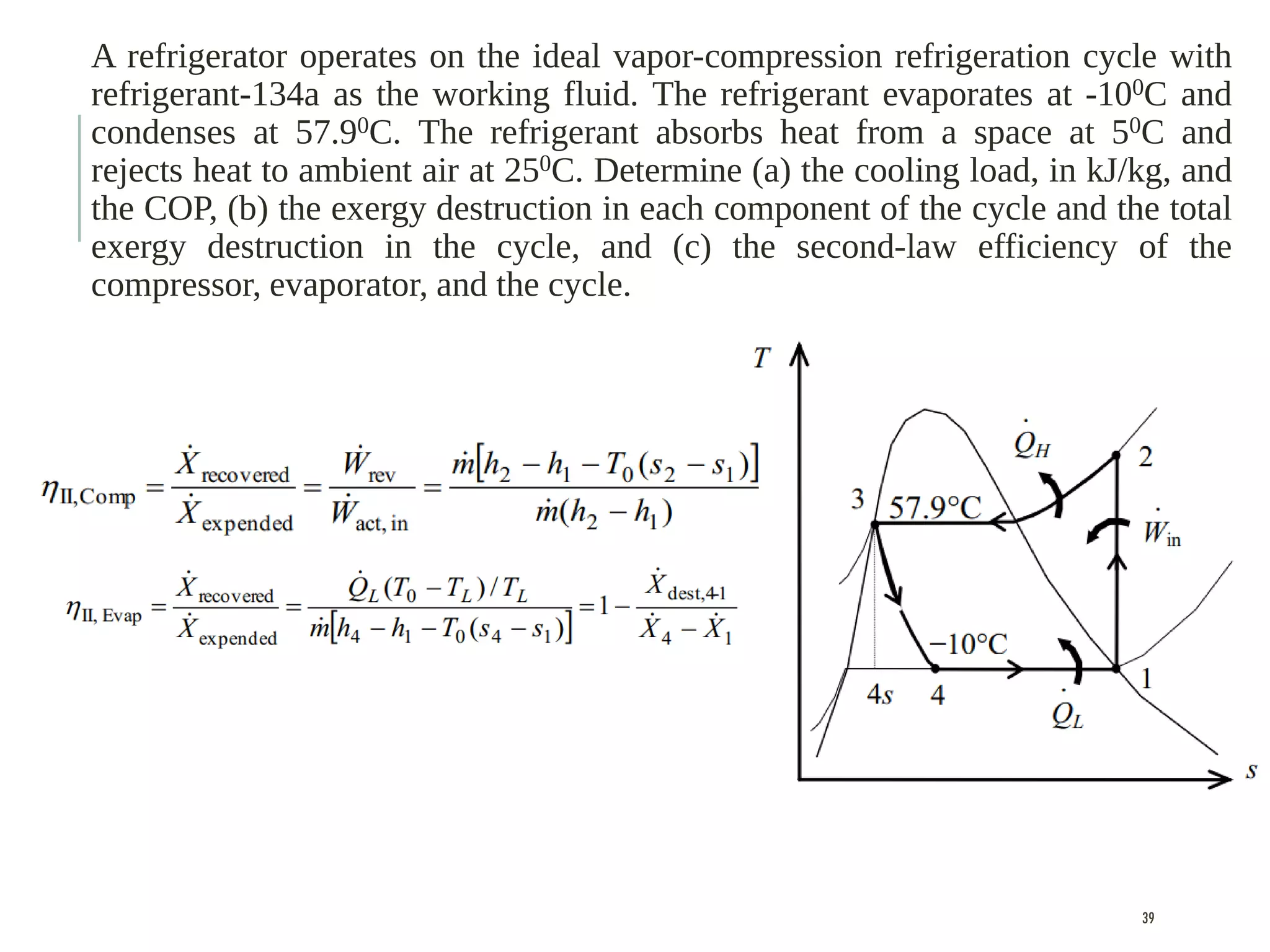
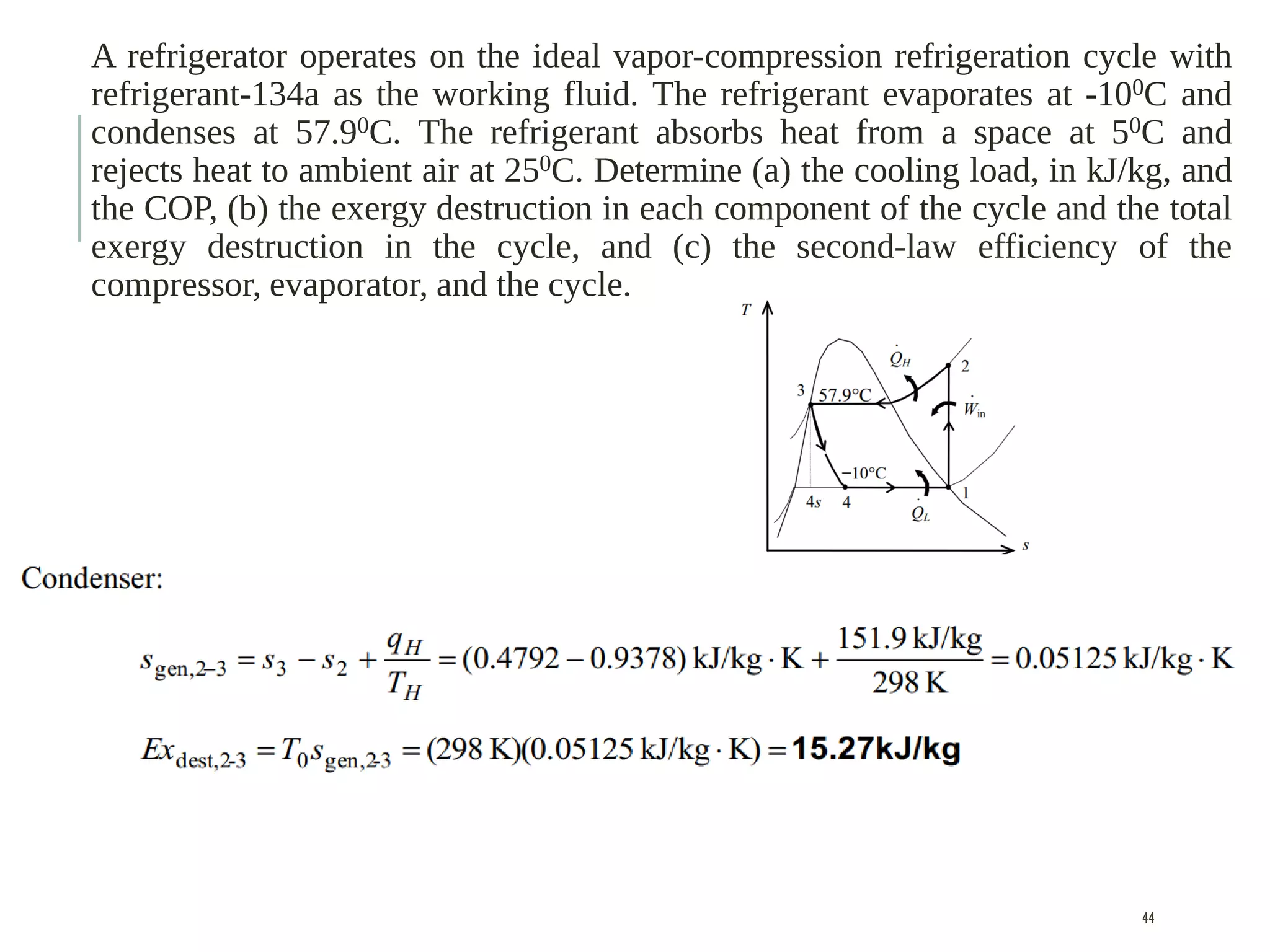
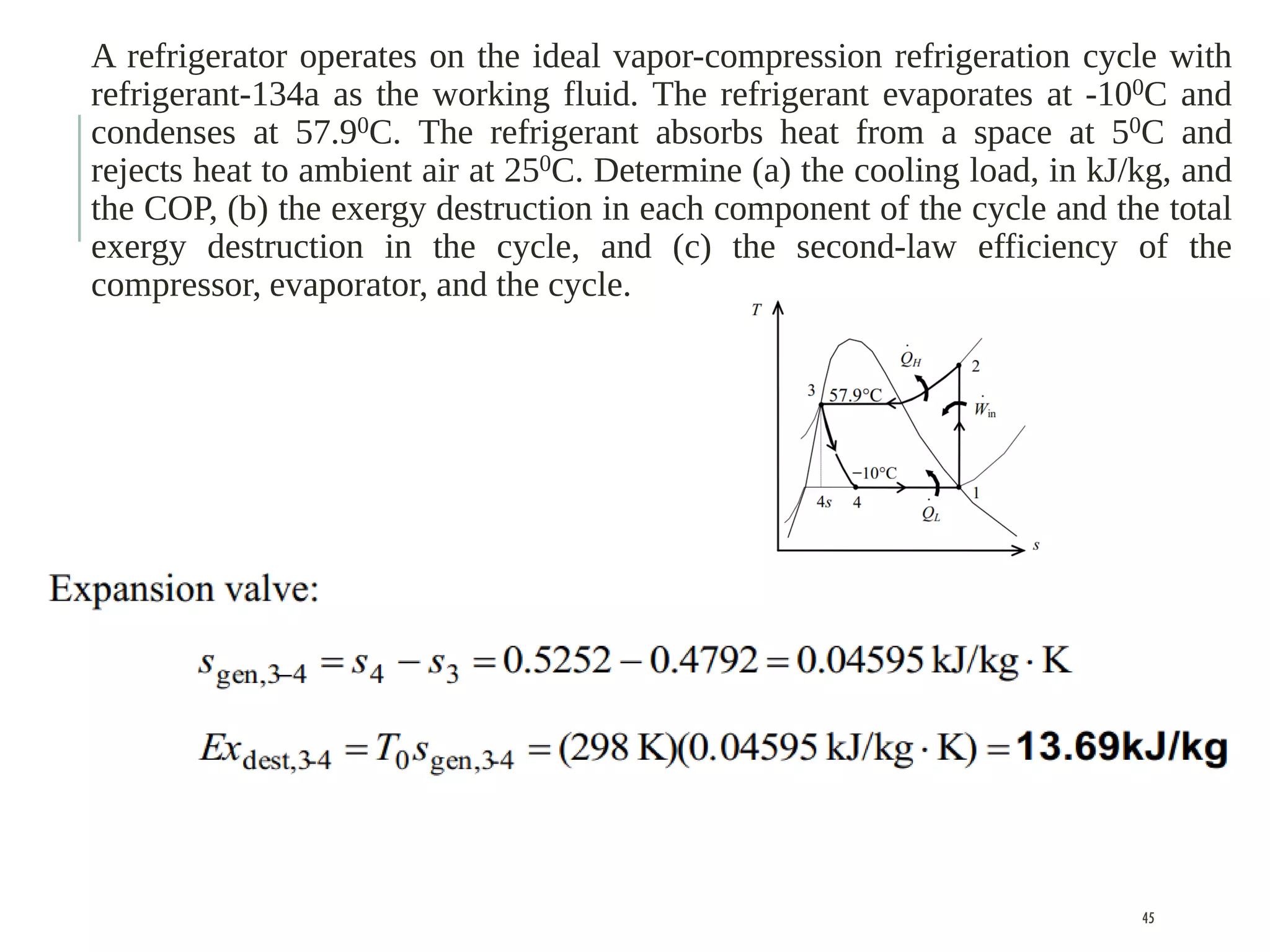
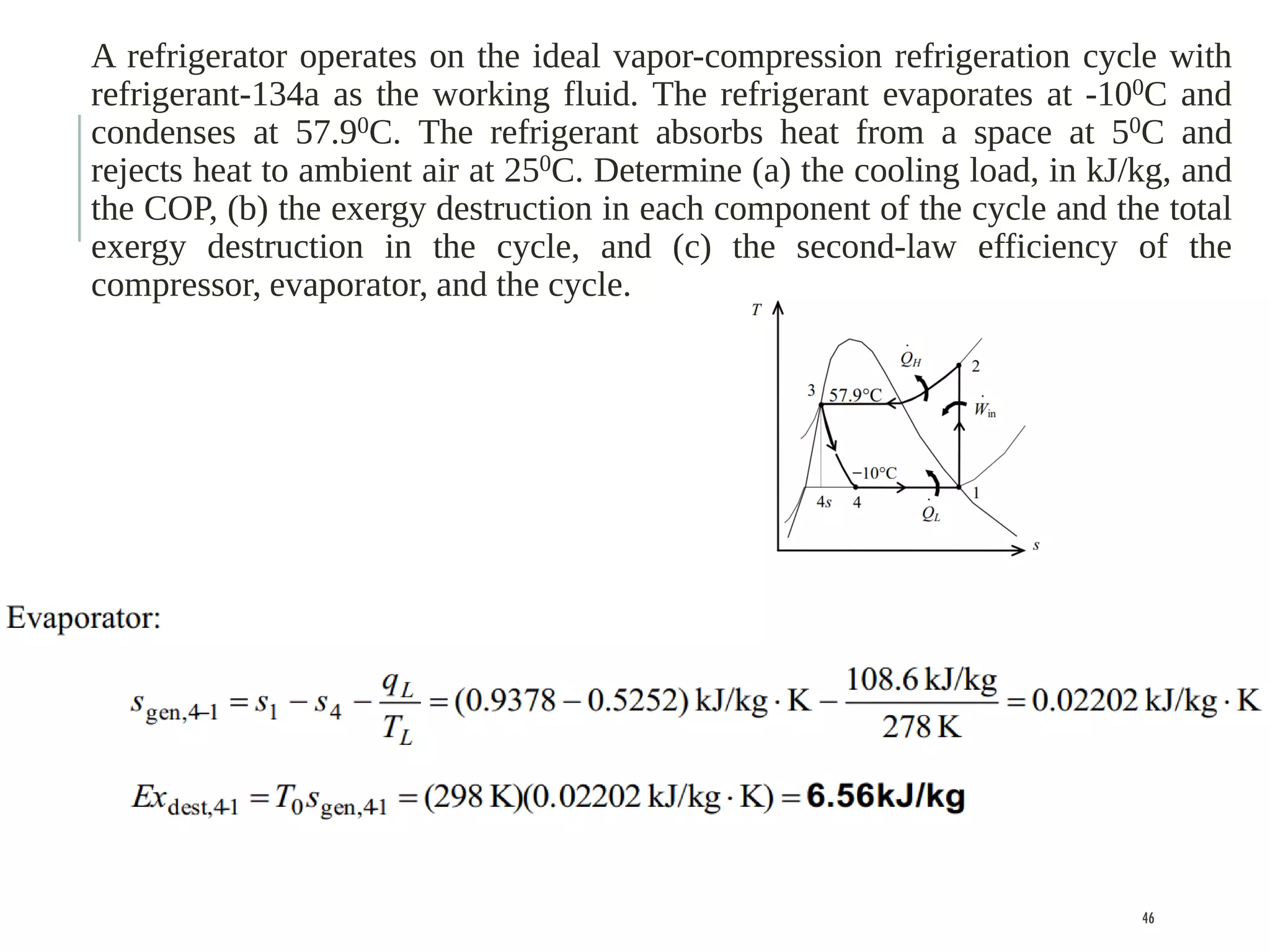
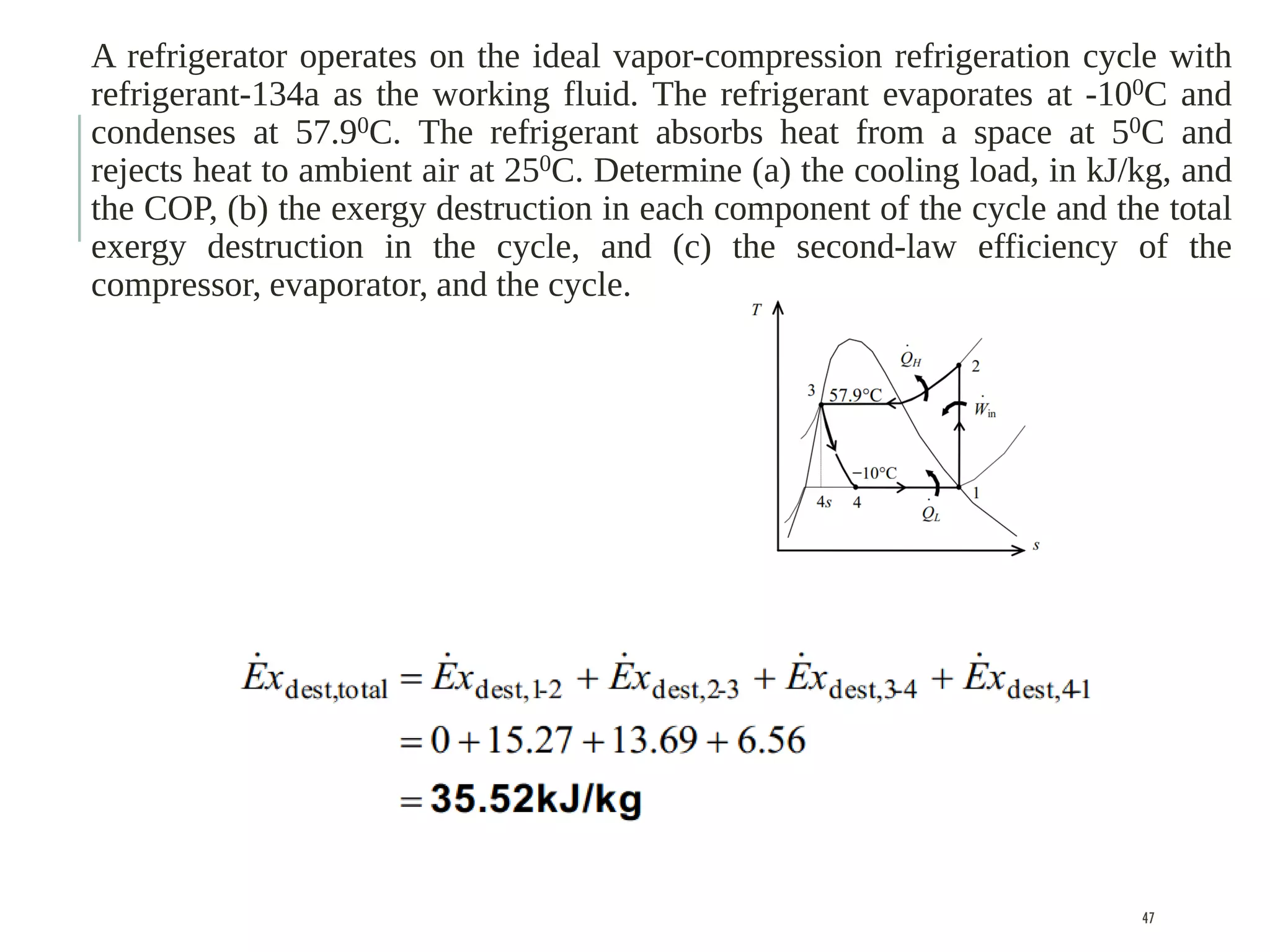
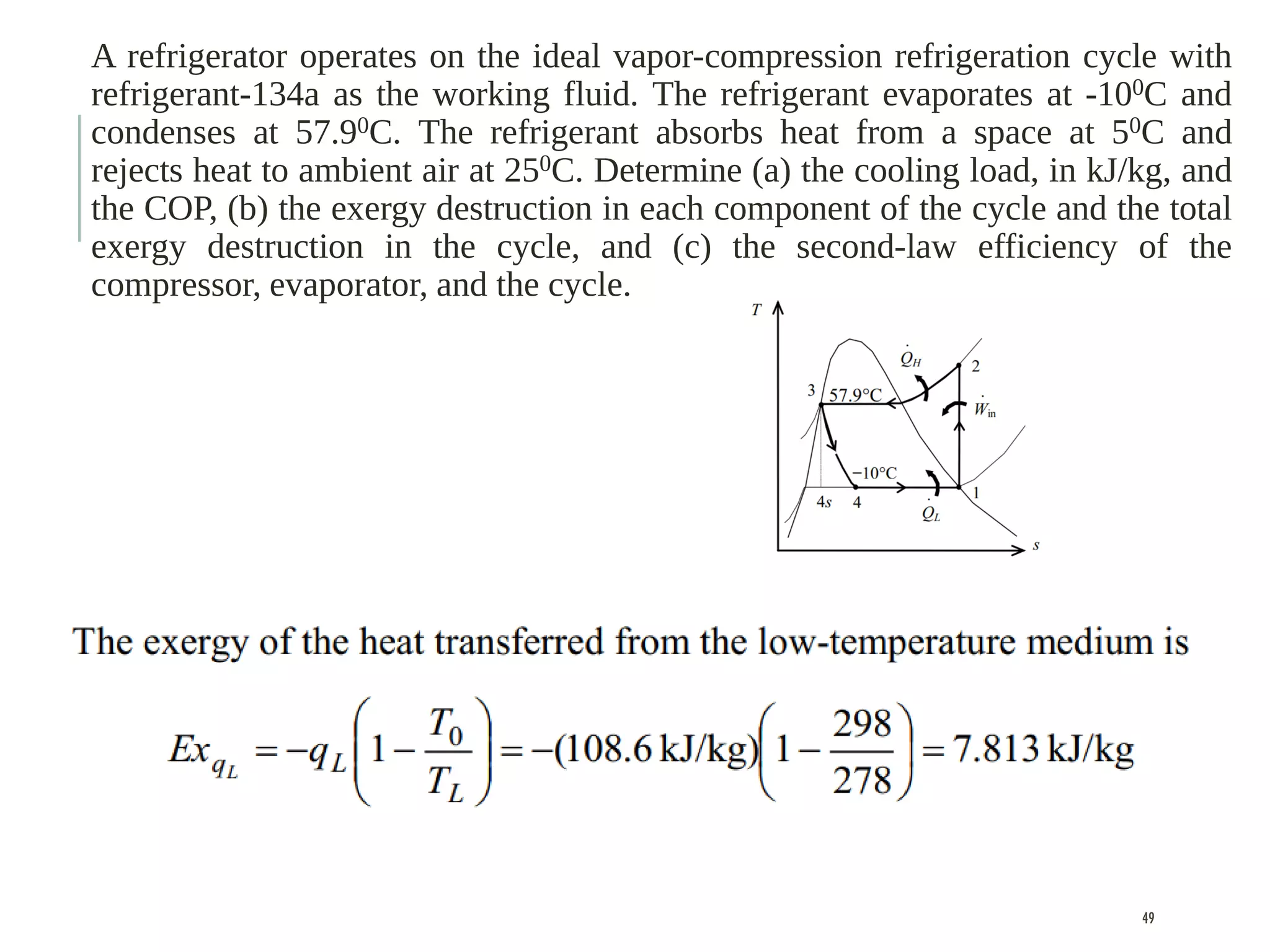
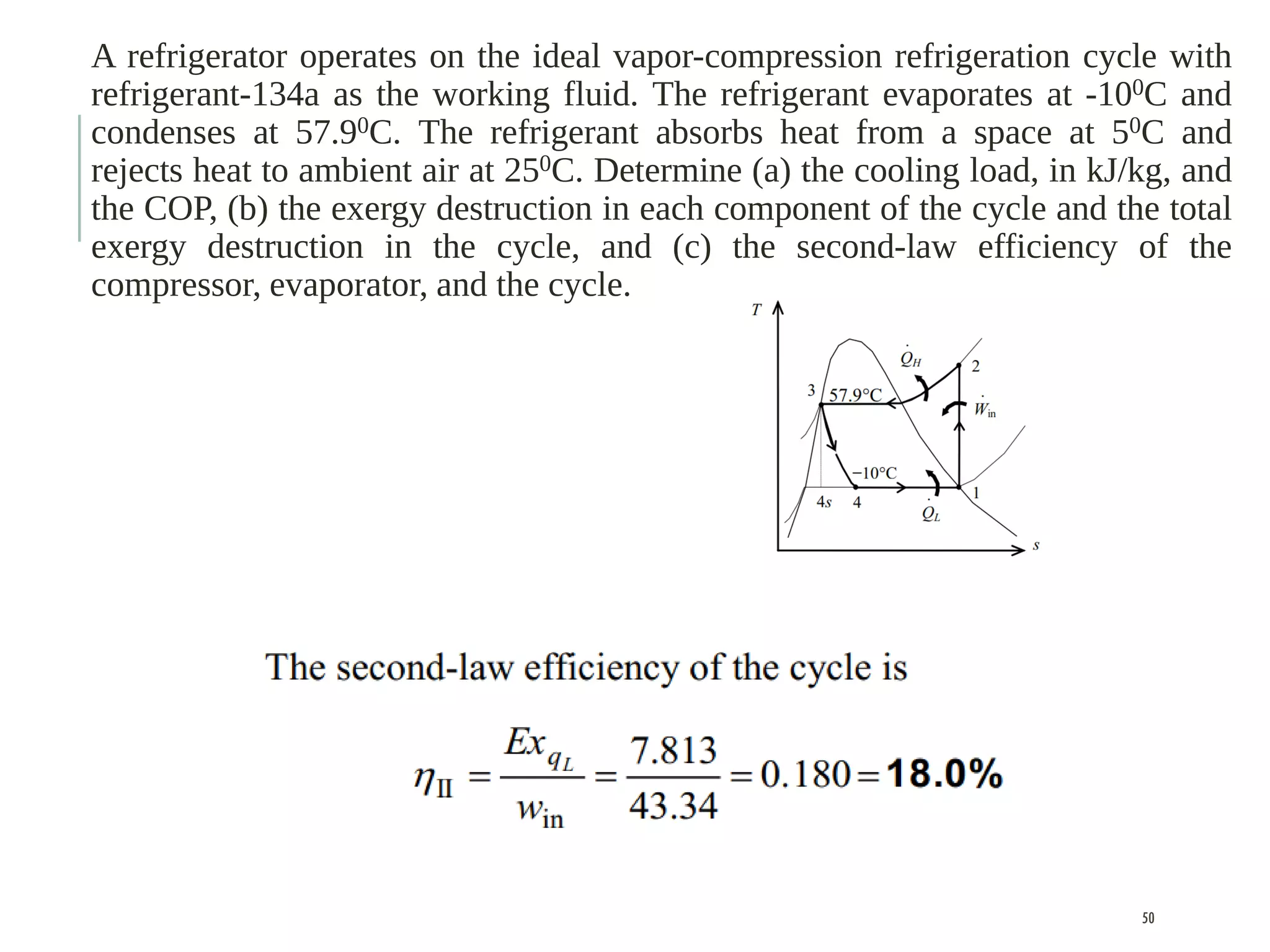
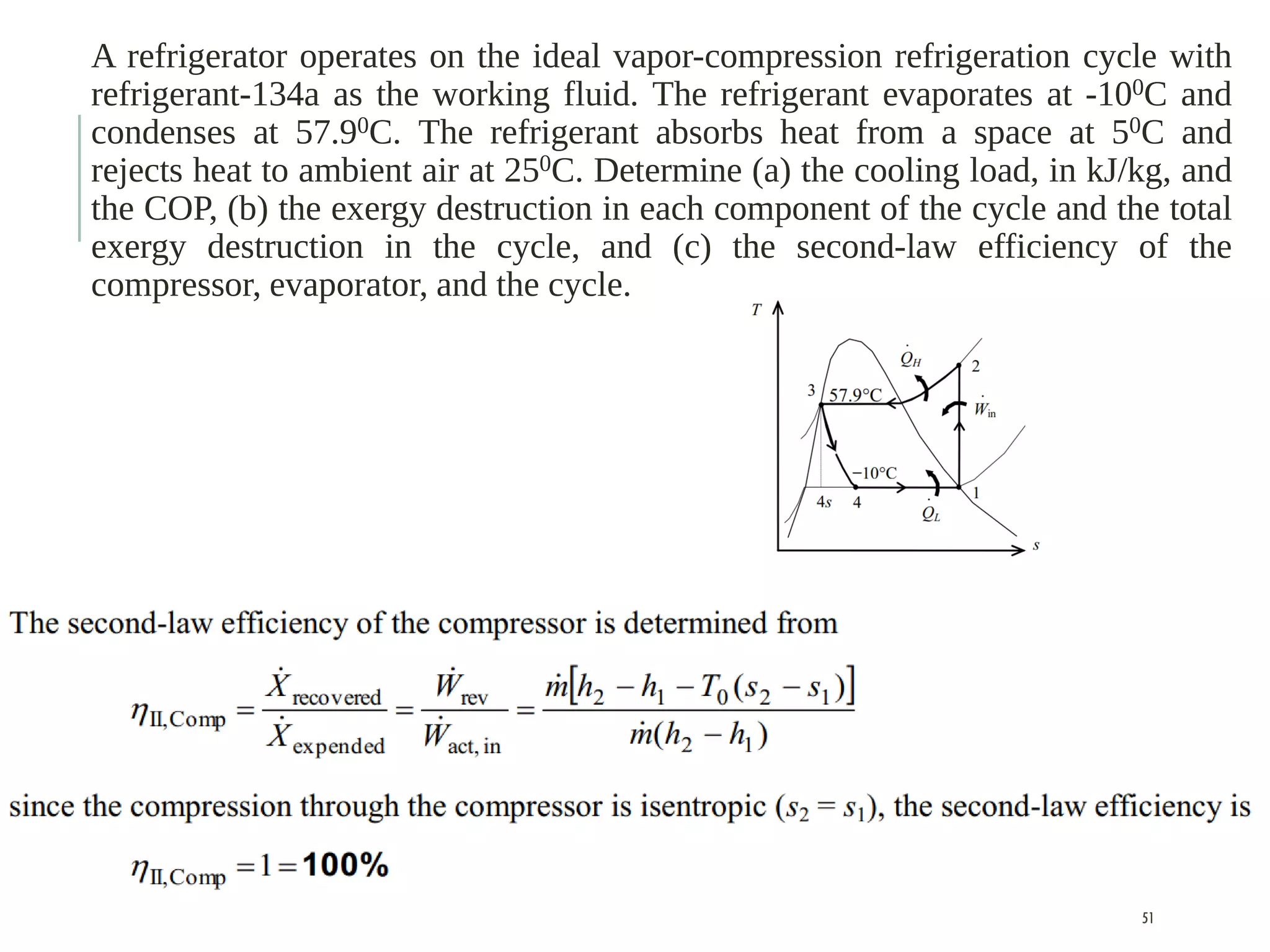
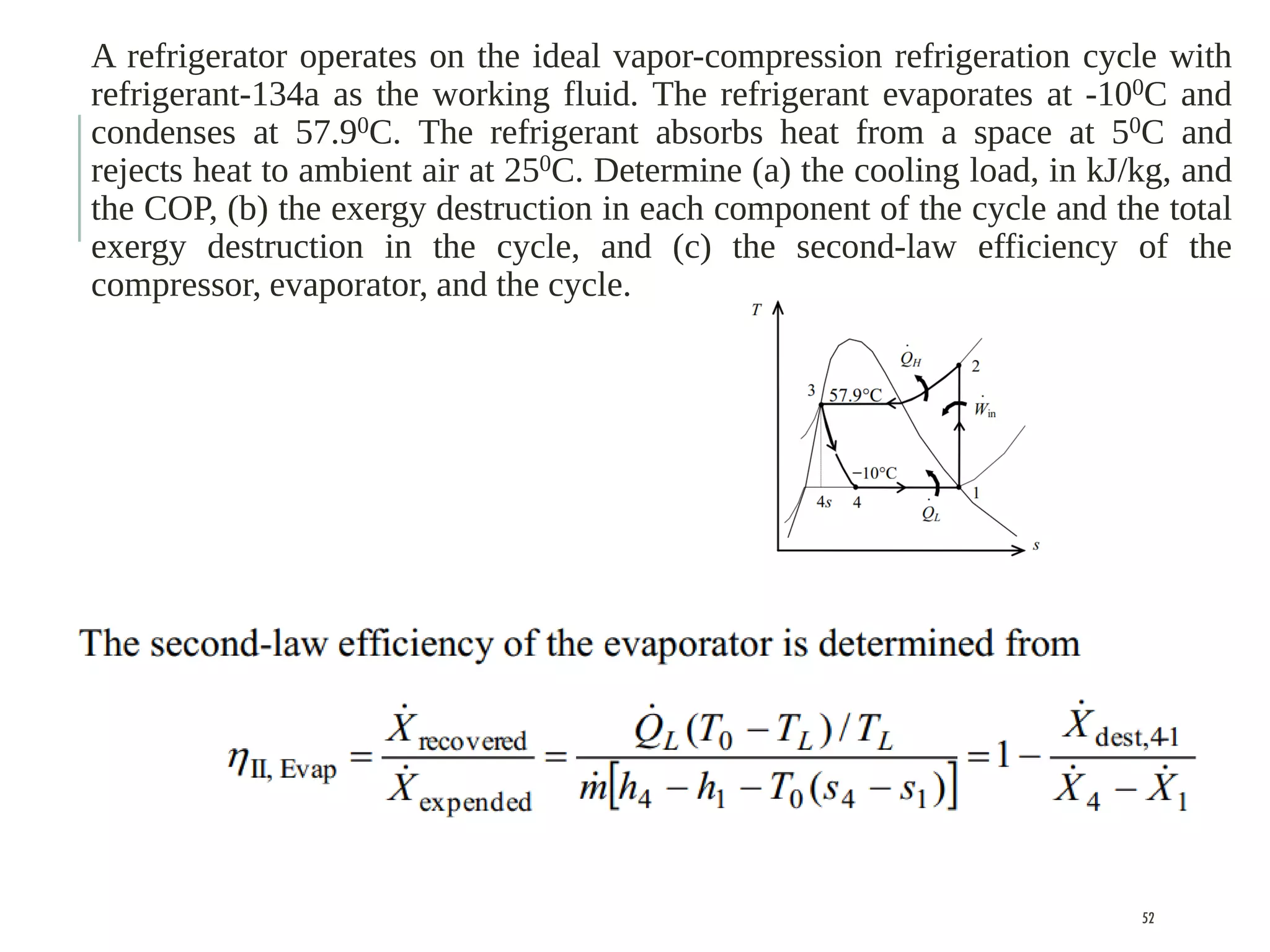
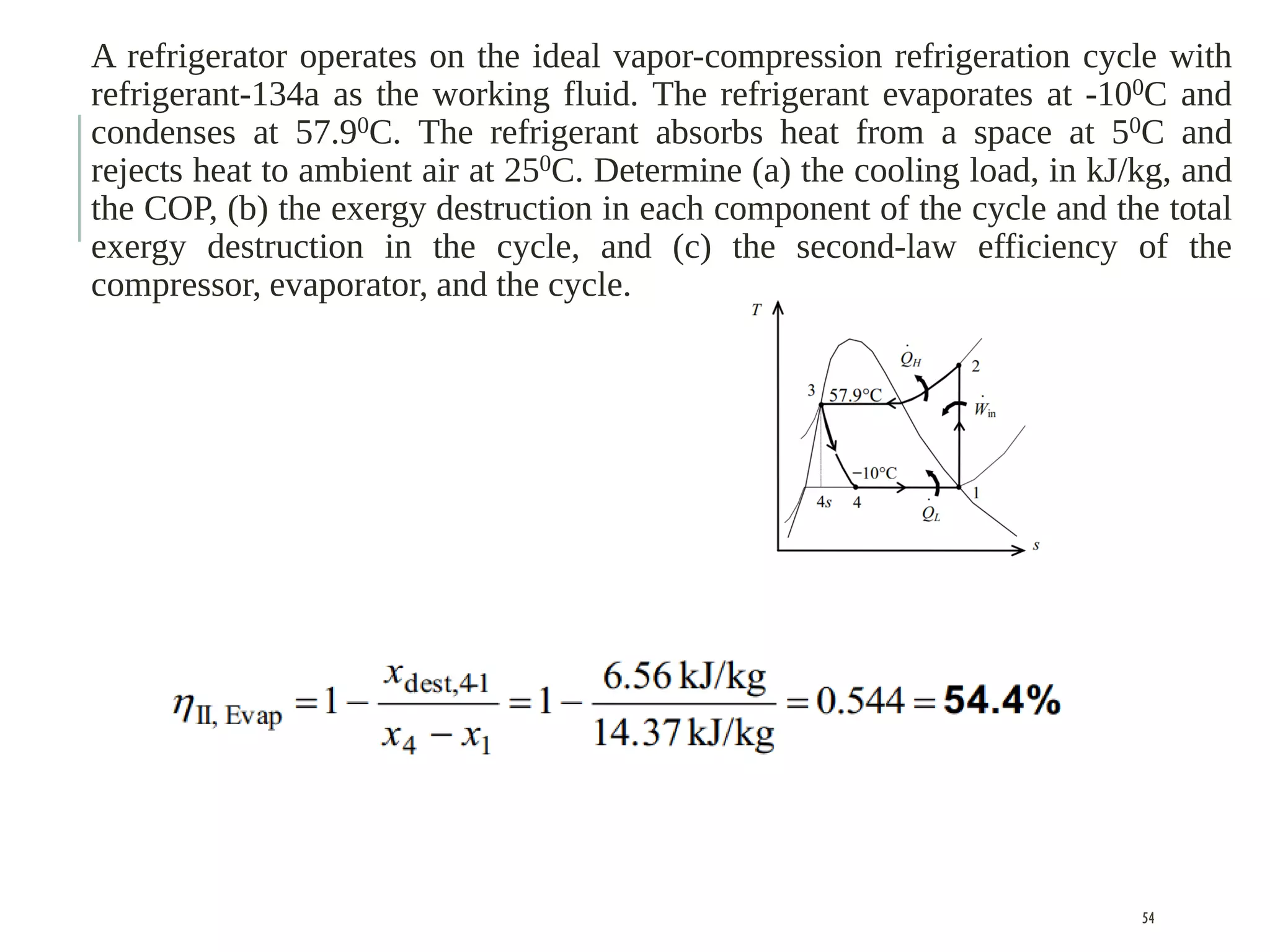
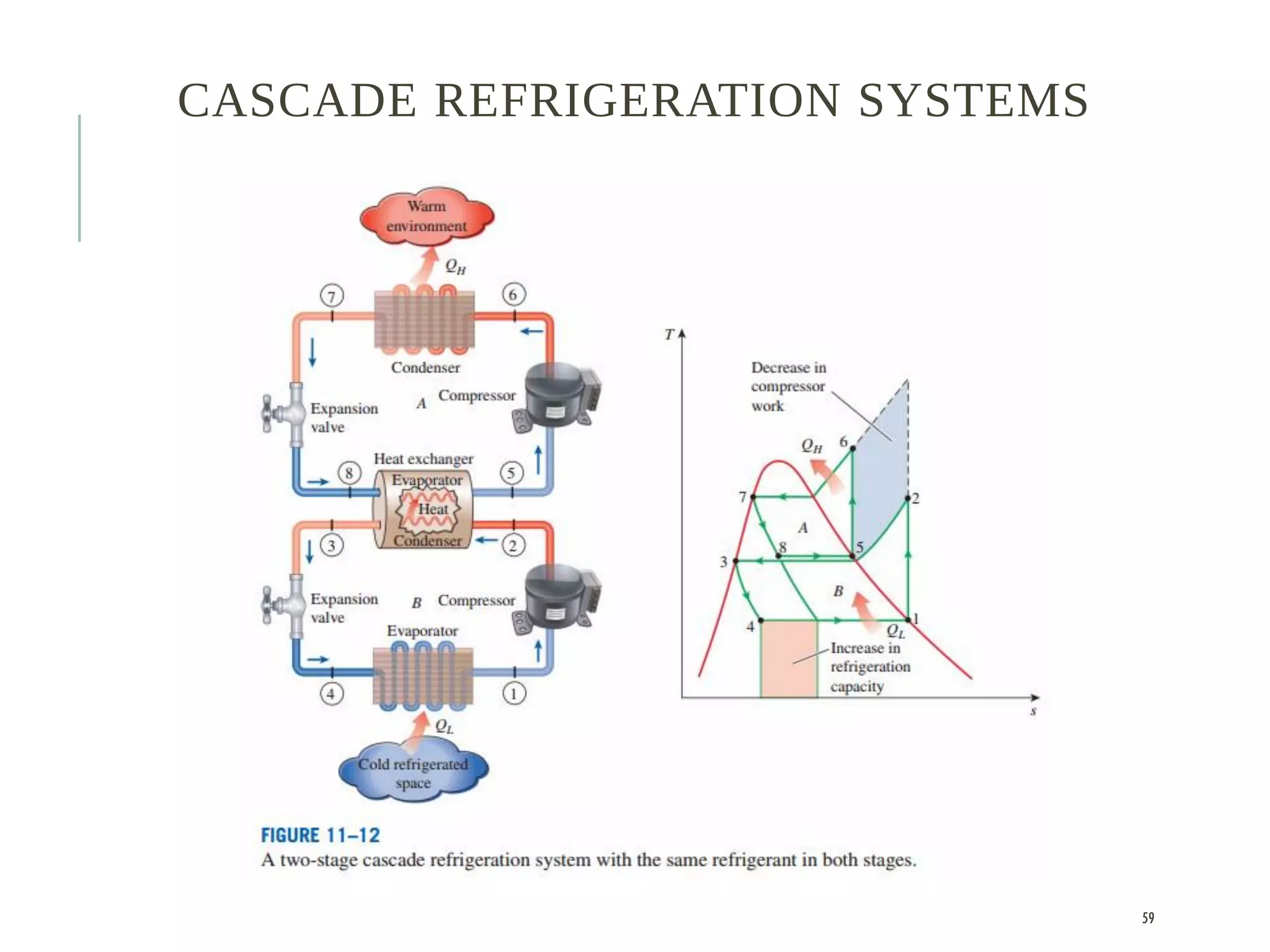
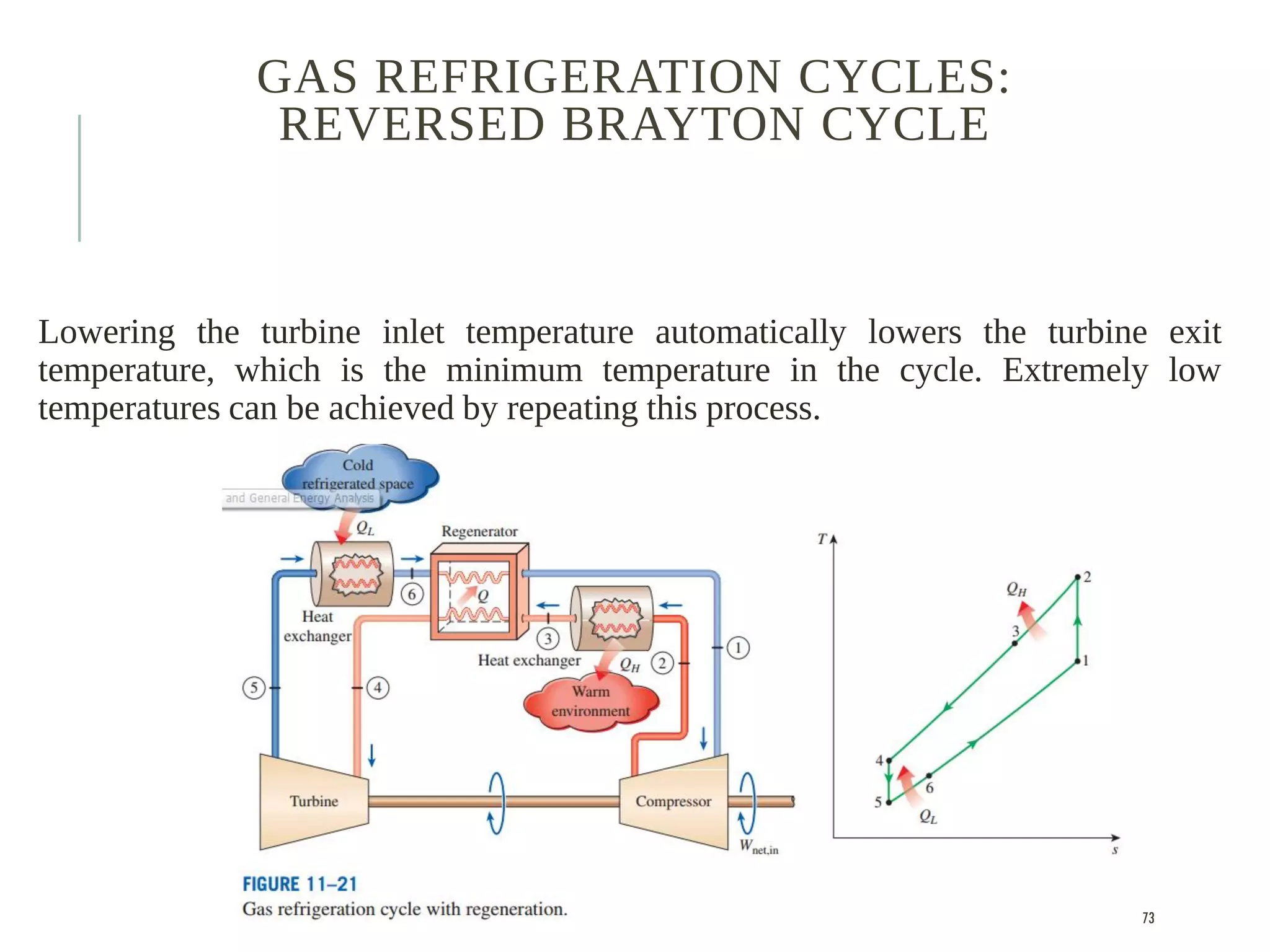
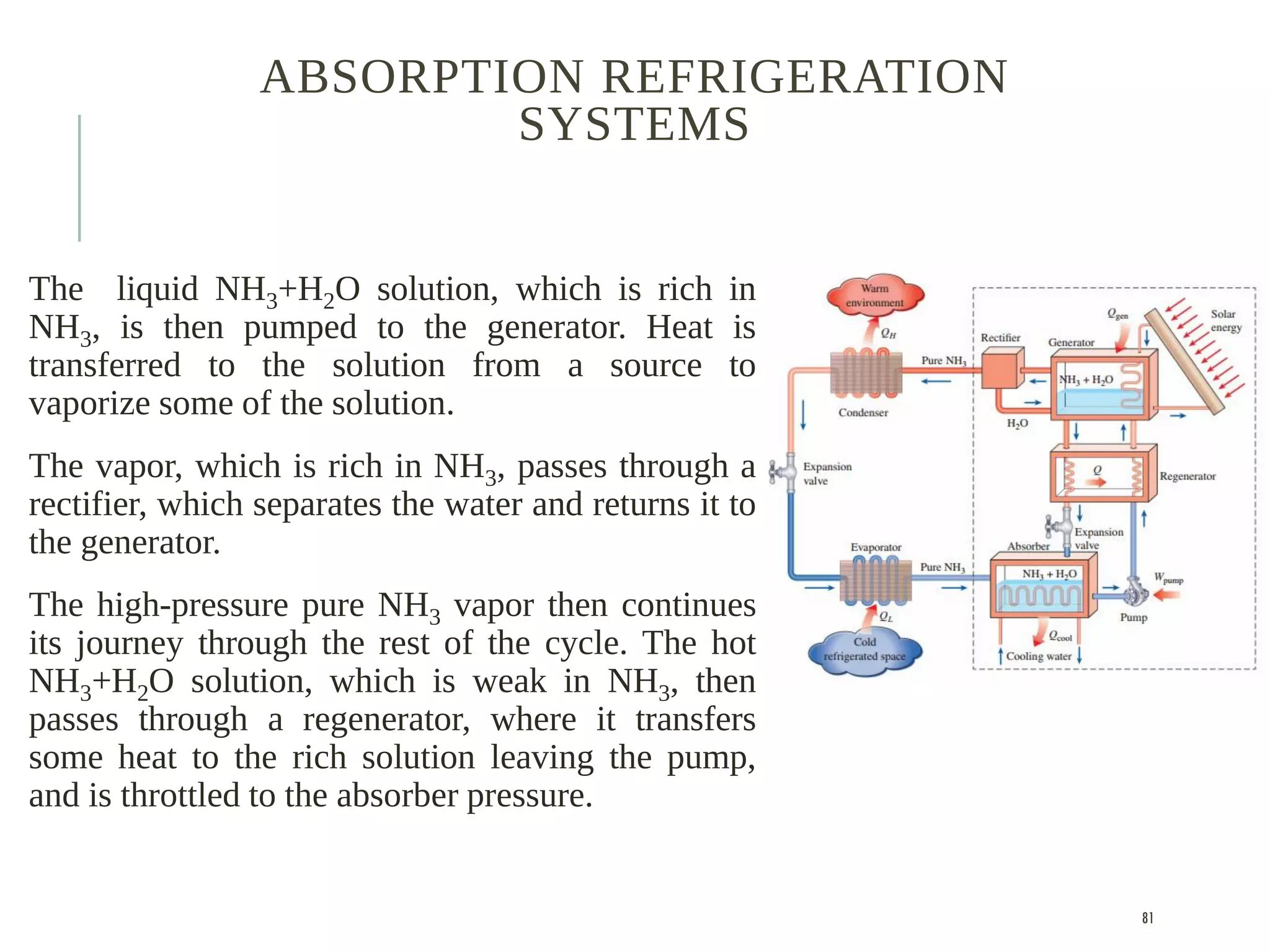
The document discusses refrigeration cycles and the ideal vapor-compression refrigeration cycle. It describes how refrigerators and heat pumps work and defines key concepts like the coefficient of performance and ton of refrigeration. The ideal vapor-compression refrigeration cycle is explained through T-s and P-h diagrams. Differences between ideal and actual vapor-compression cycles are also covered, including superheating and subcooling of the refrigerant.