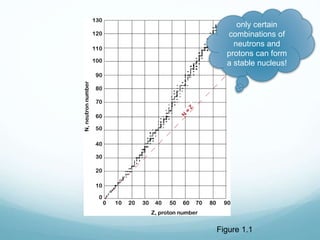
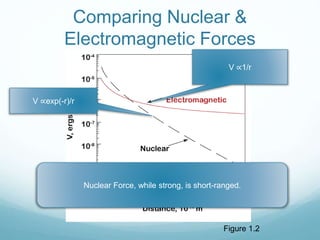
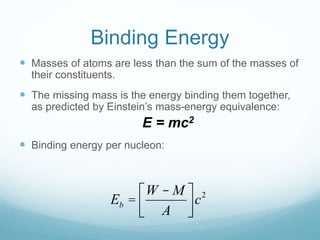
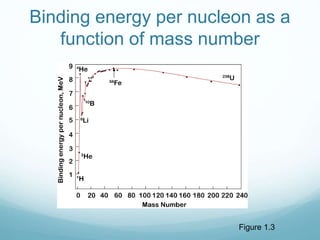
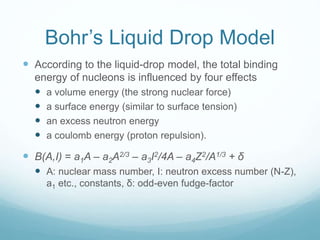
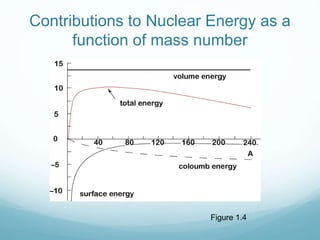
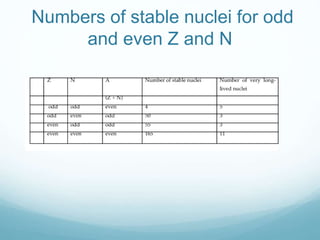
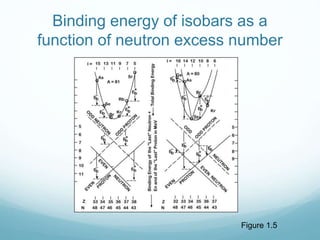
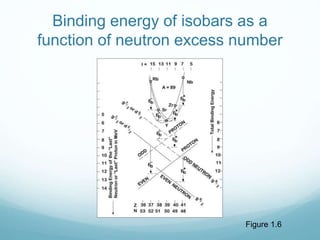
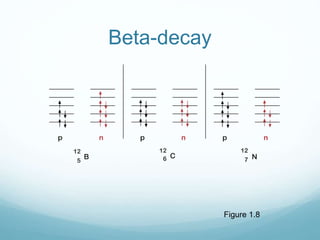
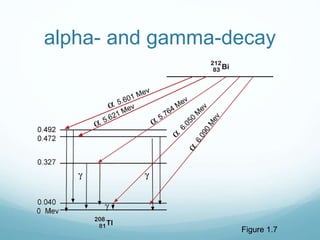
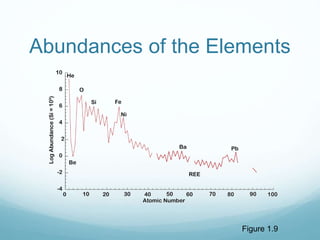
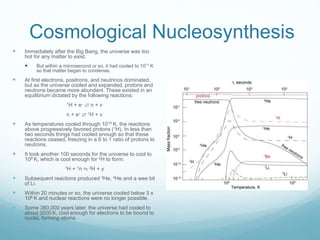
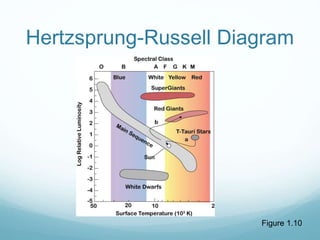
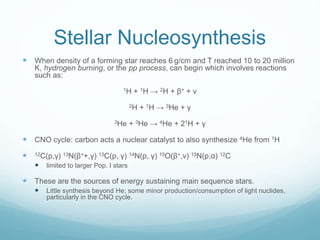
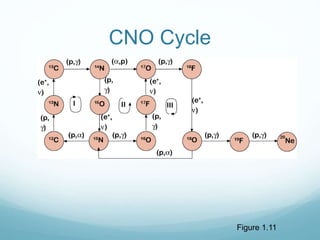
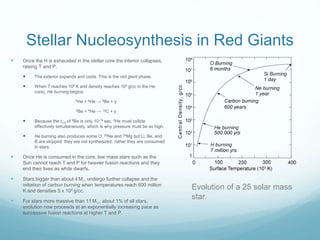
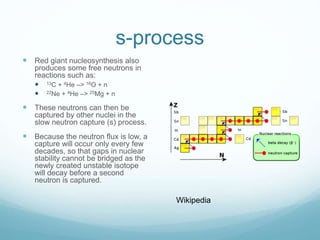
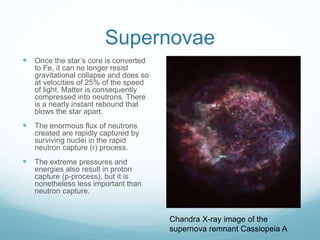
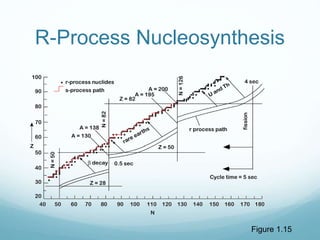
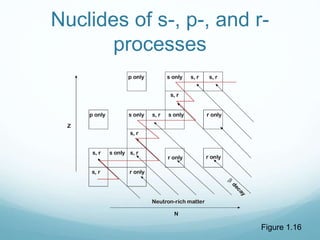
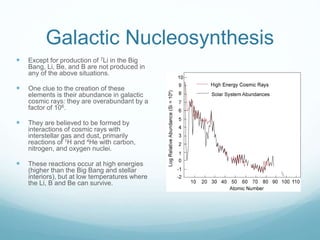
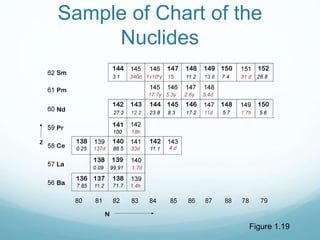
Isotope geochemistry can be used for geochronology, understanding solar system and continent evolution, tracing the history of life, unraveling climate history, and determining the origin of mineral and energy resources. The history of isotope geochemistry began with the discovery of radioactivity and production of the first radiometric age for the Earth. Isotopes have differing numbers of neutrons. Nuclear stability depends on the balance of nuclear forces. Radioactive decay follows exponential laws. Isotope ratios are altered by geological processes and can be used to study earth systems. Stable and radioactive isotopes are produced via stellar nucleosynthesis during the lives of stars and their deaths as supernovae.