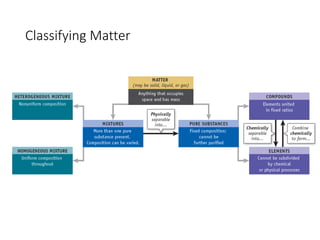
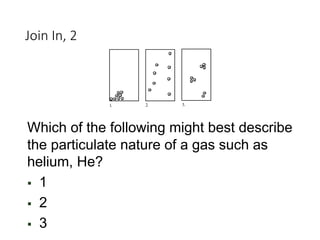
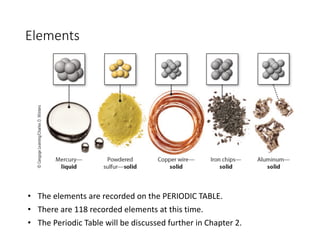
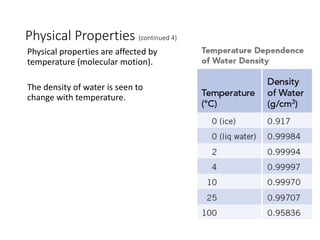
This presentation serves as the foundational cornerstone for the study of general chemistry. Titled "Module 1: Basic Concepts," it introduces learners to the essential principles and terminologies that underpin all subsequent topics in chemistry. Designed to equip students with a strong conceptual framework, this module breaks down the seemingly complex world of atoms, molecules, and matter into accessible, digestible lessons.
The presentation begins with an overview of chemistry as a central science, highlighting its connections to biology, physics, earth sciences, and environmental studies. It emphasizes the relevance of chemistry in daily life and various industries, from healthcare and agriculture to engineering and consumer products.