Ch 05 Thermodynamics (1).ppt
•Download as PPT, PDF•
1 like•37 views
Thermodynamics describes the transfer of energy and the natural tendency of systems to move towards lower energy states. A system and its surroundings are defined, and changes in a system are associated with energy transfer. Gibbs free energy (G) is a measure of a system's chemical energy and all systems naturally move towards minimum G states. G depends on enthalpy (H), temperature (T), and entropy (S). The phase or reaction with lowest G is the most stable under a given set of conditions. Phase diagrams can be constructed using G to predict stability fields with changing P and T.
Report
Share
Report
Share
Recommended
Ch 05 Thermodynamics.ppt

Thermodynamics describes the transfer of energy and the natural tendency of systems to move towards lower energy states. A system and its surroundings are defined, and changes in a system are associated with energy transfer. Systems can be in unstable, stable, or metastable energy states. Gibbs free energy (G) is a measure of a system's chemical energy and all systems naturally move towards minimum G states. G depends on enthalpy (H), temperature (T), and entropy (S). The phase with the lowest G value under a set of conditions is the most thermodynamically stable. By calculating G values, phase diagrams can be constructed to predict stability fields with changing temperature and pressure.
Gibbs Free Energy.ppt

Gibbs free energy (G) is a measure of chemical energy that can be used to determine the direction of chemical reactions and the equilibrium of products and reactants. G depends on the enthalpy (H) and entropy (S) of the system according to the equation G = H - TS. A reaction will proceed in the direction that lowers the Gibbs free energy and will reach equilibrium when the Gibbs free energies of products and reactants are equal. The change in Gibbs free energy (ΔG) can be calculated from the standard Gibbs free energies of formation (ΔG°f) of products and reactants.
lec 2 dr. marwa.ppsx

This document discusses Gibbs free energy (G) and how it relates to chemical reactions and equilibrium. It defines G and explains how it depends on enthalpy (H), entropy (S), pressure (P), and temperature (T). It describes how the change in G for a reaction (DG) determines whether the reaction is spontaneous. It shows how DG can be used to calculate equilibrium constants (K) at different temperatures and how K values change with temperature.
Capitulo 3 del libro TERMODINAMICA

Thermodynamics of Biological Systems
The chapter outlines key thermodynamic concepts including:
1) Systems can exchange energy but not matter (closed systems) or both energy and matter (open systems)
2) The first law relates changes in internal energy (ΔE) to heat (q) and work (w)
3) The second law introduces entropy (S) as a measure of disorder
4) Gibbs free energy (ΔG) determines spontaneity and is related to ΔH and TΔS
The chapter discusses coupled processes that drive unfavorable reactions, energy transduction via ATP and NADPH, and high-energy molecules like phosphates. Hydrolysis of high-energy bonds
Thermodynamics kinetics

1) Thermodynamics describes how changes in temperature, pressure, and composition affect equilibrium in rock systems. It allows prediction of mineral stability and interpretation of mineral compositions.
2) Thermodynamic models use concepts like internal energy, enthalpy, entropy, and Gibbs free energy to describe equilibrium and the direction of spontaneous processes.
3) Phase diagrams visualize thermodynamic stability by showing pressure-temperature-composition conditions where minerals are stable. However, kinetic effects can cause disequilibrium.
F y b. sc. chemical equilibria

Chemical equilibrium is briefly discussed with following topics:
Free energy change in a chemical reaction. Thermodynamic derivation of the law of chemical equilibrium.
Definition of ΔG and ΔG◦
Le Chatelier’s principle.
Relationships between Kp, Kc and Kx
C H5

This document provides an overview of key concepts in thermochemistry, including:
1) Kinetic and potential energy, and how temperature relates to the average kinetic energy of molecules. Heat is energy transferred between objects of different temperature.
2) The first law of thermodynamics states that the change in energy of a system equals the heat added plus work done. Enthalpy (H) accounts for heat and pressure-volume work.
3) Hess's law allows determining the enthalpy change of a reaction by summing the enthalpy changes of intermediate steps. Standard enthalpies of formation (ΔH°f) quantify energy released when compounds form from elements.
Revision on thermodynamics

The document discusses the first and second laws of thermodynamics. It defines key concepts like internal energy, heat, work, enthalpy, entropy, and free energies. It provides equations for calculating work, heat, changes in internal energy and enthalpy for various thermodynamic processes involving ideal gases like isothermal, isobaric, adiabatic and isochoric processes. Sample problems are also included to calculate thermodynamic parameters for processes involving gases.
Recommended
Ch 05 Thermodynamics.ppt

Thermodynamics describes the transfer of energy and the natural tendency of systems to move towards lower energy states. A system and its surroundings are defined, and changes in a system are associated with energy transfer. Systems can be in unstable, stable, or metastable energy states. Gibbs free energy (G) is a measure of a system's chemical energy and all systems naturally move towards minimum G states. G depends on enthalpy (H), temperature (T), and entropy (S). The phase with the lowest G value under a set of conditions is the most thermodynamically stable. By calculating G values, phase diagrams can be constructed to predict stability fields with changing temperature and pressure.
Gibbs Free Energy.ppt

Gibbs free energy (G) is a measure of chemical energy that can be used to determine the direction of chemical reactions and the equilibrium of products and reactants. G depends on the enthalpy (H) and entropy (S) of the system according to the equation G = H - TS. A reaction will proceed in the direction that lowers the Gibbs free energy and will reach equilibrium when the Gibbs free energies of products and reactants are equal. The change in Gibbs free energy (ΔG) can be calculated from the standard Gibbs free energies of formation (ΔG°f) of products and reactants.
lec 2 dr. marwa.ppsx

This document discusses Gibbs free energy (G) and how it relates to chemical reactions and equilibrium. It defines G and explains how it depends on enthalpy (H), entropy (S), pressure (P), and temperature (T). It describes how the change in G for a reaction (DG) determines whether the reaction is spontaneous. It shows how DG can be used to calculate equilibrium constants (K) at different temperatures and how K values change with temperature.
Capitulo 3 del libro TERMODINAMICA

Thermodynamics of Biological Systems
The chapter outlines key thermodynamic concepts including:
1) Systems can exchange energy but not matter (closed systems) or both energy and matter (open systems)
2) The first law relates changes in internal energy (ΔE) to heat (q) and work (w)
3) The second law introduces entropy (S) as a measure of disorder
4) Gibbs free energy (ΔG) determines spontaneity and is related to ΔH and TΔS
The chapter discusses coupled processes that drive unfavorable reactions, energy transduction via ATP and NADPH, and high-energy molecules like phosphates. Hydrolysis of high-energy bonds
Thermodynamics kinetics

1) Thermodynamics describes how changes in temperature, pressure, and composition affect equilibrium in rock systems. It allows prediction of mineral stability and interpretation of mineral compositions.
2) Thermodynamic models use concepts like internal energy, enthalpy, entropy, and Gibbs free energy to describe equilibrium and the direction of spontaneous processes.
3) Phase diagrams visualize thermodynamic stability by showing pressure-temperature-composition conditions where minerals are stable. However, kinetic effects can cause disequilibrium.
F y b. sc. chemical equilibria

Chemical equilibrium is briefly discussed with following topics:
Free energy change in a chemical reaction. Thermodynamic derivation of the law of chemical equilibrium.
Definition of ΔG and ΔG◦
Le Chatelier’s principle.
Relationships between Kp, Kc and Kx
C H5

This document provides an overview of key concepts in thermochemistry, including:
1) Kinetic and potential energy, and how temperature relates to the average kinetic energy of molecules. Heat is energy transferred between objects of different temperature.
2) The first law of thermodynamics states that the change in energy of a system equals the heat added plus work done. Enthalpy (H) accounts for heat and pressure-volume work.
3) Hess's law allows determining the enthalpy change of a reaction by summing the enthalpy changes of intermediate steps. Standard enthalpies of formation (ΔH°f) quantify energy released when compounds form from elements.
Revision on thermodynamics

The document discusses the first and second laws of thermodynamics. It defines key concepts like internal energy, heat, work, enthalpy, entropy, and free energies. It provides equations for calculating work, heat, changes in internal energy and enthalpy for various thermodynamic processes involving ideal gases like isothermal, isobaric, adiabatic and isochoric processes. Sample problems are also included to calculate thermodynamic parameters for processes involving gases.
Chemical Reactions.ppt

1) The document discusses two chemical reactions: reaction (1) of CH4 and O2 producing CO2 and H2O, and reaction (2) of CH4 and CO2 producing 2CO and 2H2.
2) Thermodynamic properties such as enthalpy (H) and Gibbs free energy (G) are introduced to explain why reaction (1) is exothermic and proceeds at 400K, while reaction (2) is endothermic and does not proceed at 400K.
3) The concepts of chemical equilibrium are then covered, noting that reactions may stop before reactants are fully consumed, with the ratio of products to reactants (reaction quotient) equaling the equilibrium constant (K
Thermodynamics

A homogeneous thermodynamic system is one whose properties are uniform throughout. A heterogeneous system contains distinct phases.
There are several types of thermodynamic processes including isochoric (constant volume), isobaric (constant pressure), and adiabatic (no heat transfer). Extensive properties depend on amount of substance and intensive properties do not.
The first law of thermodynamics states that energy is conserved and heat and work are equivalent. For an ideal gas undergoing an adiabatic process, PVγ is constant, where γ is the heat capacity ratio.
Ch6 z5e thermo

The document discusses key concepts in thermodynamics including:
- Energy is the ability to do work and is conserved. It exists in the forms of heat and work.
- Enthalpy (H) is a state function that is the sum of a system's internal energy (E) and pressure-volume work (PV) at constant pressure.
- The first law of thermodynamics states that energy is constant in the universe and can be calculated as the heat (q) plus work (w) transferred to or from a system.
AP_Chem_Thermodynamics.pptx

Thermodynamics is the study of energy and its transformations. Thermochemistry is the subdiscipline involving chemical reactions and energy changes. The first law of thermodynamics states that energy is conserved as it transforms between forms. Energy exists in kinetic and potential forms. Kinetic energy is associated with motion, while potential energy is stored energy due to position or composition. Heat is the transfer of kinetic energy between objects of different temperatures until they reach equilibrium. Exothermic processes release heat from a system, while endothermic processes absorb heat into a system. Enthalpy changes (ΔH) quantify the heat absorbed or released by chemical reactions. Spontaneous processes are those that proceed without outside intervention, as dictated by a negative change
Plotting of different parameters entropy, enthalpy, gibbs free energy, heat c...

This document discusses various thermodynamic concepts including entropy, enthalpy, Gibbs free energy, heat capacity, and their relationships with temperature. It provides equations relating these concepts and explains how to calculate slopes from plots of these variables. Specifically, it states that the slope of a temperature vs entropy graph equals the system's temperature. It also discusses how phase changes occur based on the relative free energy of different phases and how entropy affects phase stability at different temperatures.
Thermodynamics relations

This document discusses thermodynamic properties and relations. Some key points:
- Thermodynamic properties that cannot be directly measured must be related to measurable properties.
- Properties are continuous point functions that have exact differentials and can be written as functions of two independent variables like z(x,y).
- The Maxwell relations relate the partial derivatives of properties like pressure, specific volume, temperature and entropy.
- The Clapeyron equation relates the enthalpy change of phase change to the slope of the saturation curve on a pressure-temperature diagram.
- Specific heats, internal energy, enthalpy and entropy changes can be expressed in terms of pressure, specific volume, temperature and specific he
thermodynamicsrelations-161231100305.pdf

This document discusses thermodynamic properties and relations. Some key points:
- Thermodynamic properties can be measured directly or related through equations based on properties being point functions specified by two intensive properties.
- Maxwell relations relate partial derivatives of properties like pressure, specific volume, temperature and entropy for simple compressible substances.
- The Clapeyron equation relates enthalpy change during a phase change to the slope of the saturation curve on a pressure-temperature diagram.
- Relations are developed to express changes in internal energy, enthalpy and entropy in terms of pressure, specific volume, temperature and specific heats for real gases.
Chemical Engineering desciption, laws.ppt

1) The document provides an introduction to thermodynamics concepts including definitions of intensive and extensive variables, the first and second laws of thermodynamics, and Gibbs free energy.
2) It discusses how thermodynamics can be used to determine the stability of phases and reactions between phases in geological systems.
3) Gibbs free energy can be used to predict the most stable configuration of phases by finding the lowest absolute Gibbs free energy or by finding the conditions where the Gibbs free energy is equal between phases.
1 handout.ppt

1) The document provides an introduction to thermodynamics concepts including definitions of intensive and extensive variables, the first and second laws of thermodynamics, and Gibbs free energy.
2) It discusses how thermodynamics can be used to determine the stability of phases and reactions between phases in geological systems.
3) Gibbs free energy can be used to predict the most stable configuration of phases by finding the lowest absolute Gibbs free energy, or to find reactions between phases by determining where the Gibbs free energy is equal between configurations.
Causes of change

The document defines key thermodynamic terms like heat, work, internal energy, temperature, enthalpy, and heat capacity. It distinguishes between heat and temperature. It discusses specific heat and molar heat capacity, and provides values for common substances. It describes how to calculate heat transfer and enthalpy change using specific heat, molar heat capacity, and temperature change. Hess's law and standard enthalpies of formation are also explained for calculating enthalpy changes in chemical reactions.
CHAPTER 6- CHEMICAL REACTIAN EQUILIBRIA.PPT

This chapter discusses chemical reaction equilibria, focusing on how temperature, pressure, and initial composition affect the equilibrium conversions of chemical reactions. It introduces the reaction coordinate, which characterizes the extent of a reaction, and explains how the numbers of moles and mole fractions of reaction species relate to the coordinate. It also covers the equilibrium constant K, how it depends on temperature based on the standard Gibbs energy change, and how to evaluate K values from thermodynamic data.
Clean ch06 lecture_7e

This document provides an overview of thermochemistry and calorimetry. It discusses:
1) Thermochemistry deals with the heat involved in chemical and physical changes. Energy can be transferred between a system and its surroundings as heat or work.
2) Enthalpy (H) is a measure of the total energy of a system at constant pressure. For reactions that do not involve large volume changes, change in enthalpy (ΔH) can approximate change in energy (ΔE).
3) Calorimetry involves measuring heat transfer during chemical or physical changes using devices like coffee cup calorimeters. The heat transferred (q) can be calculated using specific heat capacity (c), mass (
Honour Chemistry Unit 4 Thermoc.docx

This document provides an overview of thermochemistry and nuclear chemistry concepts. It defines different types of energy, including radiant, thermal, chemical, and potential energy. The first law of thermodynamics states that energy cannot be created or destroyed, only converted from one form to another. Chemical reactions can be exothermic or endothermic depending on whether energy is released or absorbed. Enthalpy changes (ΔH) are used to quantify energy changes in chemical and physical processes. The document also discusses thermochemical concepts like heat, work, state functions, and calorimetry which involve measuring energy changes during physical and chemical changes.
aula-9.ppt

This document discusses entropy and the second law of thermodynamics. It introduces entropy as a property and defines entropy transfer and production. Equations are derived relating entropy change to heat transfer (TdS equations) for closed systems undergoing reversible processes. These equations allow calculating entropy changes between any two states of a pure substance or ideal gas. The document also discusses using temperature-entropy (T-s) and enthalpy-entropy (h-s) diagrams to analyze thermodynamic processes and calculate entropy changes.
Lesson 2.pptx

The document discusses heat transfer during phase changes. It defines specific heat as the heat required to change an object's temperature, and latent heat as the heat required to change an object's phase, such as from solid to liquid. Latent heat of fusion is the energy required for melting/freezing, while latent heat of vaporization is for boiling/condensing. Two examples problems are included to calculate the energy required to melt silver and the amount of water that remains unfrozen given a specific heat transfer.
4 PCh Lecture.ppt

The document discusses Gibbs free energy and its relationship to chemical equilibrium. It defines Gibbs free energy and how it can be used to predict the spontaneity of reactions. The Gibbs free energy change, ΔG, is related to the standard Gibbs free energy change, ΔG°, and the reaction quotient, Q, by the equation ΔG = ΔG° + RTlnQ. At equilibrium when ΔG = 0, Q equals the equilibrium constant, K. Expressions are provided for calculating K for different types of reactions, such as gas phase, liquid phase, and liquid-solid reactions. The key points are that a reaction will only proceed spontaneously if ΔG is negative and that a reaction will reach equilibrium when ΔG equals
Ch6 Thermochemistry (updated)

Thermochemistry is the study of heat changes in chemical reactions. There are several types of energy including chemical, thermal, nuclear, and radiant energy. Heat is the transfer of thermal energy between objects at different temperatures. Thermochemistry examines heat absorbed or released by chemical reactions using concepts like exothermic, endothermic, enthalpy, and calorimetry. The first law of thermodynamics states that energy cannot be created or destroyed, only transferred between systems and their surroundings.
Clausius-clapeyron Equation

The document discusses the Clausius-Clapeyron equation, which relates the rate of change of vapor pressure or melting point with temperature to the latent heat of vaporization, fusion, or sublimation. It presents the derivation of the equation based on an infinitely small Carnot cycle involving a two-phase system. The document then applies the Clausius-Clapeyron equation to analyze the relationships between pressure, temperature, and phase transitions for vaporization, melting, and sublimation.
Thermodynamic ppt

B.Sc II Sem III Chemistry
College Name :- Late Ku.Durga K.Banmeru Science College Lonar
University Name:- Sant Gadgebaba Amravati University Amravati
More Related Content
Similar to Ch 05 Thermodynamics (1).ppt
Chemical Reactions.ppt

1) The document discusses two chemical reactions: reaction (1) of CH4 and O2 producing CO2 and H2O, and reaction (2) of CH4 and CO2 producing 2CO and 2H2.
2) Thermodynamic properties such as enthalpy (H) and Gibbs free energy (G) are introduced to explain why reaction (1) is exothermic and proceeds at 400K, while reaction (2) is endothermic and does not proceed at 400K.
3) The concepts of chemical equilibrium are then covered, noting that reactions may stop before reactants are fully consumed, with the ratio of products to reactants (reaction quotient) equaling the equilibrium constant (K
Thermodynamics

A homogeneous thermodynamic system is one whose properties are uniform throughout. A heterogeneous system contains distinct phases.
There are several types of thermodynamic processes including isochoric (constant volume), isobaric (constant pressure), and adiabatic (no heat transfer). Extensive properties depend on amount of substance and intensive properties do not.
The first law of thermodynamics states that energy is conserved and heat and work are equivalent. For an ideal gas undergoing an adiabatic process, PVγ is constant, where γ is the heat capacity ratio.
Ch6 z5e thermo

The document discusses key concepts in thermodynamics including:
- Energy is the ability to do work and is conserved. It exists in the forms of heat and work.
- Enthalpy (H) is a state function that is the sum of a system's internal energy (E) and pressure-volume work (PV) at constant pressure.
- The first law of thermodynamics states that energy is constant in the universe and can be calculated as the heat (q) plus work (w) transferred to or from a system.
AP_Chem_Thermodynamics.pptx

Thermodynamics is the study of energy and its transformations. Thermochemistry is the subdiscipline involving chemical reactions and energy changes. The first law of thermodynamics states that energy is conserved as it transforms between forms. Energy exists in kinetic and potential forms. Kinetic energy is associated with motion, while potential energy is stored energy due to position or composition. Heat is the transfer of kinetic energy between objects of different temperatures until they reach equilibrium. Exothermic processes release heat from a system, while endothermic processes absorb heat into a system. Enthalpy changes (ΔH) quantify the heat absorbed or released by chemical reactions. Spontaneous processes are those that proceed without outside intervention, as dictated by a negative change
Plotting of different parameters entropy, enthalpy, gibbs free energy, heat c...

This document discusses various thermodynamic concepts including entropy, enthalpy, Gibbs free energy, heat capacity, and their relationships with temperature. It provides equations relating these concepts and explains how to calculate slopes from plots of these variables. Specifically, it states that the slope of a temperature vs entropy graph equals the system's temperature. It also discusses how phase changes occur based on the relative free energy of different phases and how entropy affects phase stability at different temperatures.
Thermodynamics relations

This document discusses thermodynamic properties and relations. Some key points:
- Thermodynamic properties that cannot be directly measured must be related to measurable properties.
- Properties are continuous point functions that have exact differentials and can be written as functions of two independent variables like z(x,y).
- The Maxwell relations relate the partial derivatives of properties like pressure, specific volume, temperature and entropy.
- The Clapeyron equation relates the enthalpy change of phase change to the slope of the saturation curve on a pressure-temperature diagram.
- Specific heats, internal energy, enthalpy and entropy changes can be expressed in terms of pressure, specific volume, temperature and specific he
thermodynamicsrelations-161231100305.pdf

This document discusses thermodynamic properties and relations. Some key points:
- Thermodynamic properties can be measured directly or related through equations based on properties being point functions specified by two intensive properties.
- Maxwell relations relate partial derivatives of properties like pressure, specific volume, temperature and entropy for simple compressible substances.
- The Clapeyron equation relates enthalpy change during a phase change to the slope of the saturation curve on a pressure-temperature diagram.
- Relations are developed to express changes in internal energy, enthalpy and entropy in terms of pressure, specific volume, temperature and specific heats for real gases.
Chemical Engineering desciption, laws.ppt

1) The document provides an introduction to thermodynamics concepts including definitions of intensive and extensive variables, the first and second laws of thermodynamics, and Gibbs free energy.
2) It discusses how thermodynamics can be used to determine the stability of phases and reactions between phases in geological systems.
3) Gibbs free energy can be used to predict the most stable configuration of phases by finding the lowest absolute Gibbs free energy or by finding the conditions where the Gibbs free energy is equal between phases.
1 handout.ppt

1) The document provides an introduction to thermodynamics concepts including definitions of intensive and extensive variables, the first and second laws of thermodynamics, and Gibbs free energy.
2) It discusses how thermodynamics can be used to determine the stability of phases and reactions between phases in geological systems.
3) Gibbs free energy can be used to predict the most stable configuration of phases by finding the lowest absolute Gibbs free energy, or to find reactions between phases by determining where the Gibbs free energy is equal between configurations.
Causes of change

The document defines key thermodynamic terms like heat, work, internal energy, temperature, enthalpy, and heat capacity. It distinguishes between heat and temperature. It discusses specific heat and molar heat capacity, and provides values for common substances. It describes how to calculate heat transfer and enthalpy change using specific heat, molar heat capacity, and temperature change. Hess's law and standard enthalpies of formation are also explained for calculating enthalpy changes in chemical reactions.
CHAPTER 6- CHEMICAL REACTIAN EQUILIBRIA.PPT

This chapter discusses chemical reaction equilibria, focusing on how temperature, pressure, and initial composition affect the equilibrium conversions of chemical reactions. It introduces the reaction coordinate, which characterizes the extent of a reaction, and explains how the numbers of moles and mole fractions of reaction species relate to the coordinate. It also covers the equilibrium constant K, how it depends on temperature based on the standard Gibbs energy change, and how to evaluate K values from thermodynamic data.
Clean ch06 lecture_7e

This document provides an overview of thermochemistry and calorimetry. It discusses:
1) Thermochemistry deals with the heat involved in chemical and physical changes. Energy can be transferred between a system and its surroundings as heat or work.
2) Enthalpy (H) is a measure of the total energy of a system at constant pressure. For reactions that do not involve large volume changes, change in enthalpy (ΔH) can approximate change in energy (ΔE).
3) Calorimetry involves measuring heat transfer during chemical or physical changes using devices like coffee cup calorimeters. The heat transferred (q) can be calculated using specific heat capacity (c), mass (
Honour Chemistry Unit 4 Thermoc.docx

This document provides an overview of thermochemistry and nuclear chemistry concepts. It defines different types of energy, including radiant, thermal, chemical, and potential energy. The first law of thermodynamics states that energy cannot be created or destroyed, only converted from one form to another. Chemical reactions can be exothermic or endothermic depending on whether energy is released or absorbed. Enthalpy changes (ΔH) are used to quantify energy changes in chemical and physical processes. The document also discusses thermochemical concepts like heat, work, state functions, and calorimetry which involve measuring energy changes during physical and chemical changes.
aula-9.ppt

This document discusses entropy and the second law of thermodynamics. It introduces entropy as a property and defines entropy transfer and production. Equations are derived relating entropy change to heat transfer (TdS equations) for closed systems undergoing reversible processes. These equations allow calculating entropy changes between any two states of a pure substance or ideal gas. The document also discusses using temperature-entropy (T-s) and enthalpy-entropy (h-s) diagrams to analyze thermodynamic processes and calculate entropy changes.
Lesson 2.pptx

The document discusses heat transfer during phase changes. It defines specific heat as the heat required to change an object's temperature, and latent heat as the heat required to change an object's phase, such as from solid to liquid. Latent heat of fusion is the energy required for melting/freezing, while latent heat of vaporization is for boiling/condensing. Two examples problems are included to calculate the energy required to melt silver and the amount of water that remains unfrozen given a specific heat transfer.
4 PCh Lecture.ppt

The document discusses Gibbs free energy and its relationship to chemical equilibrium. It defines Gibbs free energy and how it can be used to predict the spontaneity of reactions. The Gibbs free energy change, ΔG, is related to the standard Gibbs free energy change, ΔG°, and the reaction quotient, Q, by the equation ΔG = ΔG° + RTlnQ. At equilibrium when ΔG = 0, Q equals the equilibrium constant, K. Expressions are provided for calculating K for different types of reactions, such as gas phase, liquid phase, and liquid-solid reactions. The key points are that a reaction will only proceed spontaneously if ΔG is negative and that a reaction will reach equilibrium when ΔG equals
Ch6 Thermochemistry (updated)

Thermochemistry is the study of heat changes in chemical reactions. There are several types of energy including chemical, thermal, nuclear, and radiant energy. Heat is the transfer of thermal energy between objects at different temperatures. Thermochemistry examines heat absorbed or released by chemical reactions using concepts like exothermic, endothermic, enthalpy, and calorimetry. The first law of thermodynamics states that energy cannot be created or destroyed, only transferred between systems and their surroundings.
Clausius-clapeyron Equation

The document discusses the Clausius-Clapeyron equation, which relates the rate of change of vapor pressure or melting point with temperature to the latent heat of vaporization, fusion, or sublimation. It presents the derivation of the equation based on an infinitely small Carnot cycle involving a two-phase system. The document then applies the Clausius-Clapeyron equation to analyze the relationships between pressure, temperature, and phase transitions for vaporization, melting, and sublimation.
Thermodynamic ppt

B.Sc II Sem III Chemistry
College Name :- Late Ku.Durga K.Banmeru Science College Lonar
University Name:- Sant Gadgebaba Amravati University Amravati
Similar to Ch 05 Thermodynamics (1).ppt (20)
Thermodynamics and laws of thermodynamics and osmotic or diffusion

Thermodynamics and laws of thermodynamics and osmotic or diffusion
Plotting of different parameters entropy, enthalpy, gibbs free energy, heat c...

Plotting of different parameters entropy, enthalpy, gibbs free energy, heat c...
More from Ser Louis Fabunan
Enzymes are biological molecules tOhat a

This document discusses enzymes and their functions. It defines enzymes as biological catalysts that facilitate chemical reactions in living organisms. It categorizes enzymes into six main types based on the reactions they catalyze - oxidoreductases, transferases, hydrolases, lyases, isomerases, and ligases. Examples are provided of specific enzymes like lactase, amylase, and lipase along with their functions. The document also describes how enzymes lower activation energy, form enzyme-substrate complexes, and are affected by substrate concentration. It explains the lock-and-key and induced fit models of enzyme-substrate binding.
TR-1.ppt

This document provides an overview of classical physics in the late 19th century, including mechanics, electromagnetism, thermodynamics, and the atomic theory of matter. It discusses outstanding questions like the structure of atoms and blackbody radiation. New discoveries like X-rays and radioactivity added complications. This set the stage for the development of modern physics theories like relativity and quantum mechanics to resolve issues classical physics could not.
bioethics_ppt.ppt

This document provides an introduction to bioethics. It defines ethics as concerning right and wrong and morality. Bioethics is important because historical events like the Tuskegee Syphilis Experiment showed that scientific progress requires ethical guidelines. The document discusses principles of bioethics like beneficence, non-maleficence, and autonomy. It also explains that ethical dilemmas have no right answers, but require reasoned debate and consideration of all perspectives to find the best solution. Developing ethical solutions takes time and open discussion rather than isolation or majority rule.
introductiontobiochemistry-161031150006.pptx

This document provides an introduction to cell structure and function and biochemistry. It defines biochemistry as the study of biological processes at the cellular and molecular level. The key components of cells are described, including the differences between prokaryotic and eukaryotic cells. The major classes of biomolecules like proteins, carbohydrates, lipids, and nucleic acids are introduced along with their functions. Common biochemical reactions like oxidation-reduction are also summarized. Maintaining the high internal order of cells requires processes like biosynthesis, transport across membranes, cell movement, and waste removal.
atmospheric pressure (1).pptx

Atmospheric pressure is measured in millibars and is caused by the weight of the air above pressing down. It varies depending on altitude, temperature, and water vapor content. Higher altitudes, higher temperatures, and more moisture all result in lower atmospheric pressure. Atmospheric pressure is measured using mercury or aneroid barometers.
3-1Air Pressure.ppt

- Air has mass and pressure due to gravity pressing down on its mass. Denser air has more pressure.
- Atmospheric pressure can be measured using barometers like mercury or aneroid barometers. Weather reports use units like inches of mercury or millibars to indicate pressure.
- Air pressure decreases with increasing altitude and elevation due to there being less air above pressing down. The higher the altitude, the less dense the air becomes.
earthsciencepptx-151128011727-lva1-app6891 (1).pdf

This document provides an overview of Earth science and the solar system. It describes the four branches of Earth science: geology, meteorology, astronomy, and oceanography. It then explains the importance of studying Earth science, including understanding natural resources and hazards. The document continues by defining the solar system and describing how it formed based on the planetesimal and nebular theories. It outlines the layers of the Sun and solar activities like sunspots and solar flares. Finally, it characterizes the eight major planets, grouping the inner terrestrial planets and outer gas giants, and provides key details about each planetary body.
Community-Practices.pptx

This document discusses communities of practice and their importance in community development. It defines a community of practice as a group that shares a common interest or goal and interacts regularly to better achieve these. Key characteristics include a shared domain of interest, joint activities and relationship building among members. Communities of practice are important as they educate members, support collaboration, cultivate learning, encourage sharing, and help integrate new knowledge. The values of community development that communities of practice support include collaboration, meaningful participation, respect, strengths-based approaches, and integrity.
mendeliangenetics-200827064849.pptx

This document provides an overview of Mendelian genetics. It discusses:
- Gregor Mendel, the father of genetics, who discovered the basic principles of heredity through pea plant experiments in the mid-1800s.
- Key genetic terminology like traits, heredity, genetics, alleles, genotype and phenotype.
- Mendel's experiments on monohybrid and dihybrid crosses, which showed that traits are inherited in predictable ratios and follow the laws of dominance and segregation.
- Mendel's laws of inheritance: dominance, segregation, and independent assortment. His work established the foundation of modern genetics.
NATIONAL LEARNING CAMP__Specific Guidelines FINAL.pdf

The document outlines the Department of Education's agenda to improve learner outcomes and teacher competence through a voluntary three to five week summer break program called the National Learning Camp (NLC). It provides templates for the NLC including a registration form, parent consent form, and suggested timetables. It also discusses providing updates to parents on student progress and assessing students before and after the program. The document details monitoring and evaluation of the NLC's implementation where school heads supervise schools, regional offices monitor compliance, and the central office evaluates results to improve the policy.
Bulacan-Strategic-Plan-2013-2014.pptx

This document provides a situational analysis and proposed strategies, programs, and projects for the Division of Bulacan for school years 2014-2017. Key points from the analysis include high enrollment rates but low test scores, and high dropout rates. Proposed strategies address these issues through interventions to reduce dropout rates, livelihood programs to address poverty, and technical assistance for teachers. The financial plan outlines funding sources from the provincial SEF for initiatives related to curriculum/instruction, school governance, private school support, and student/teacher development.
The-Act-of-Proclamation-of-Independence-Day.pptx

The document discusses key events surrounding the Philippine Declaration of Independence from Spanish colonial rule on June 12, 1898. It provides background on the Philippine Revolution against Spain led by national heroes like Emilio Aguinaldo, Andres Bonifacio, and Jose Rizal. On June 12th, Aguinaldo proclaimed Philippine independence and established the First Philippine Republic. The declaration marked the end of over 300 years of Spanish colonialism and the beginning of Philippine sovereignty.
Planning Instruction-140907065815-phpapp01.pptx

The document discusses various aspects of instructional planning for teaching. It covers types of instructional planning like course planning, unit planning, and lesson planning. It also discusses developing objectives, designing lessons, assessing learning, and teaching strategies like lectures, discussions, demonstrations and role playing. Effective instructional planning provides direction for teachers, develops well-organized learning experiences, and prepares students for classroom activities.
104--WhatIsLiterature.ppt

The document defines the key traits and elements of literature. It discusses how literature has structure and form, explores genres like tragedy and comedy, and elements such as plot, theme, and characterization. The document contrasts popular works with those considered Literature, noting that Literature has lasting significance by providing insight into the human condition and making readers think in a way that teaches them about culture and life.
Elements Of DramaEDI.ppt

This document discusses the key elements of drama, including plot, character, setting, conflict, and theme. It explains the differences between stories and plays, such as plays being performed live on stage without a narrator. The document also covers different genres like tragedy and comedy, as well as dramatic techniques including dramatic irony, foils, monologues, soliloquies, and asides. Technical elements of drama production are also defined, such as scenery, costumes, lighting, and sound.
ILC_Overview_Presentation_2014.pptx

This document discusses the Instructional Learning Cycle (ILC), which is a repeatable process for planning and improving instruction. The ILC involves setting learning targets, planning instructional strategies, implementing lessons, analyzing student performance data, and making adjustments to improve effectiveness. Completing this course will make users familiar with the ILC phases and able to implement them in a collaborative team to continuously improve instruction aligned to standards and better meet student needs.
The Female Reproductive System.ppt

During fetal development, germ cells in the ovaries develop into oogonia which then become primary oocytes. By birth around 1 million primordial follicles exist, each containing an immature oocyte surrounded by a single layer of cells. At puberty hormone stimulation causes a few hundred follicles to mature each month, with only one oocyte being ovulated. If fertilization does not occur, the corpus luteum regresses and menstruation begins. The female reproductive system includes ovaries, fallopian tubes, uterus, cervix, and vagina which work together to allow fertilization and implantation if pregnancy occurs.
PHYS632_C1_22_Charge.ppt

1) The document provides an overview of the topics covered in the first lecture of a graduate-level electromagnetism course, including demonstrations on charging objects through friction and induction, and measuring charge.
2) Key concepts discussed are the atomic model of charge, methods of charging objects, quantization of charge, Coulomb's law, and the principle of superposition.
3) A variety of demonstrations are described, such as using a Van de Graaff generator, electroscopes, and charged conducting spheres to illustrate these fundamental electrostatics concepts.
More from Ser Louis Fabunan (20)
Dance is a multifaceted art form that combines rhythm, movement, and expressi...

Dance is a multifaceted art form that combines rhythm, movement, and expressi...
earthsciencepptx-151128011727-lva1-app6891 (1).pdf

earthsciencepptx-151128011727-lva1-app6891 (1).pdf
NATIONAL LEARNING CAMP__Specific Guidelines FINAL.pdf

NATIONAL LEARNING CAMP__Specific Guidelines FINAL.pdf
Recently uploaded
Main Java[All of the Base Concepts}.docx

This is part 1 of my Java Learning Journey. This Contains Custom methods, classes, constructors, packages, multithreading , try- catch block, finally block and more.
Hindi varnamala | hindi alphabet PPT.pdf

हिंदी वर्णमाला पीपीटी, hindi alphabet PPT presentation, hindi varnamala PPT, Hindi Varnamala pdf, हिंदी स्वर, हिंदी व्यंजन, sikhiye hindi varnmala, dr. mulla adam ali, hindi language and literature, hindi alphabet with drawing, hindi alphabet pdf, hindi varnamala for childrens, hindi language, hindi varnamala practice for kids, https://www.drmullaadamali.com
ANATOMY AND BIOMECHANICS OF HIP JOINT.pdf

it describes the bony anatomy including the femoral head , acetabulum, labrum . also discusses the capsule , ligaments . muscle that act on the hip joint and the range of motion are outlined. factors affecting hip joint stability and weight transmission through the joint are summarized.
The simplified electron and muon model, Oscillating Spacetime: The Foundation...

Discover the Simplified Electron and Muon Model: A New Wave-Based Approach to Understanding Particles delves into a groundbreaking theory that presents electrons and muons as rotating soliton waves within oscillating spacetime. Geared towards students, researchers, and science buffs, this book breaks down complex ideas into simple explanations. It covers topics such as electron waves, temporal dynamics, and the implications of this model on particle physics. With clear illustrations and easy-to-follow explanations, readers will gain a new outlook on the universe's fundamental nature.
BBR 2024 Summer Sessions Interview Training

Qualitative research interview training by Professor Katrina Pritchard and Dr Helen Williams
How to Setup Warehouse & Location in Odoo 17 Inventory

In this slide, we'll explore how to set up warehouses and locations in Odoo 17 Inventory. This will help us manage our stock effectively, track inventory levels, and streamline warehouse operations.
Your Skill Boost Masterclass: Strategies for Effective Upskilling

Your Skill Boost Masterclass: Strategies for Effective UpskillingExcellence Foundation for South Sudan
Strategies for Effective Upskilling is a presentation by Chinwendu Peace in a Your Skill Boost Masterclass organisation by the Excellence Foundation for South Sudan on 08th and 09th June 2024 from 1 PM to 3 PM on each day.PCOS corelations and management through Ayurveda.

This presentation includes basic of PCOS their pathology and treatment and also Ayurveda correlation of PCOS and Ayurvedic line of treatment mentioned in classics.
How to Add Chatter in the odoo 17 ERP Module

In Odoo, the chatter is like a chat tool that helps you work together on records. You can leave notes and track things, making it easier to talk with your team and partners. Inside chatter, all communication history, activity, and changes will be displayed.
How to Build a Module in Odoo 17 Using the Scaffold Method

Odoo provides an option for creating a module by using a single line command. By using this command the user can make a whole structure of a module. It is very easy for a beginner to make a module. There is no need to make each file manually. This slide will show how to create a module using the scaffold method.
LAND USE LAND COVER AND NDVI OF MIRZAPUR DISTRICT, UP

This Dissertation explores the particular circumstances of Mirzapur, a region located in the
core of India. Mirzapur, with its varied terrains and abundant biodiversity, offers an optimal
environment for investigating the changes in vegetation cover dynamics. Our study utilizes
advanced technologies such as GIS (Geographic Information Systems) and Remote sensing to
analyze the transformations that have taken place over the course of a decade.
The complex relationship between human activities and the environment has been the focus
of extensive research and worry. As the global community grapples with swift urbanization,
population expansion, and economic progress, the effects on natural ecosystems are becoming
more evident. A crucial element of this impact is the alteration of vegetation cover, which plays a
significant role in maintaining the ecological equilibrium of our planet.Land serves as the foundation for all human activities and provides the necessary materials for
these activities. As the most crucial natural resource, its utilization by humans results in different
'Land uses,' which are determined by both human activities and the physical characteristics of the
land.
The utilization of land is impacted by human needs and environmental factors. In countries
like India, rapid population growth and the emphasis on extensive resource exploitation can lead
to significant land degradation, adversely affecting the region's land cover.
Therefore, human intervention has significantly influenced land use patterns over many
centuries, evolving its structure over time and space. In the present era, these changes have
accelerated due to factors such as agriculture and urbanization. Information regarding land use and
cover is essential for various planning and management tasks related to the Earth's surface,
providing crucial environmental data for scientific, resource management, policy purposes, and
diverse human activities.
Accurate understanding of land use and cover is imperative for the development planning
of any area. Consequently, a wide range of professionals, including earth system scientists, land
and water managers, and urban planners, are interested in obtaining data on land use and cover
changes, conversion trends, and other related patterns. The spatial dimensions of land use and
cover support policymakers and scientists in making well-informed decisions, as alterations in
these patterns indicate shifts in economic and social conditions. Monitoring such changes with the
help of Advanced technologies like Remote Sensing and Geographic Information Systems is
crucial for coordinated efforts across different administrative levels. Advanced technologies like
Remote Sensing and Geographic Information Systems
9
Changes in vegetation cover refer to variations in the distribution, composition, and overall
structure of plant communities across different temporal and spatial scales. These changes can
occur natural.
Chapter 4 - Islamic Financial Institutions in Malaysia.pptx

Chapter 4 - Islamic Financial Institutions in Malaysia.pptxMohd Adib Abd Muin, Senior Lecturer at Universiti Utara Malaysia
This slide is special for master students (MIBS & MIFB) in UUM. Also useful for readers who are interested in the topic of contemporary Islamic banking.
Exploiting Artificial Intelligence for Empowering Researchers and Faculty, In...

Exploiting Artificial Intelligence for Empowering Researchers and Faculty, In...Dr. Vinod Kumar Kanvaria
Exploiting Artificial Intelligence for Empowering Researchers and Faculty,
International FDP on Fundamentals of Research in Social Sciences
at Integral University, Lucknow, 06.06.2024
By Dr. Vinod Kumar KanvariaCommunity pharmacy- Social and preventive pharmacy UNIT 5

Covered community pharmacy topic of the subject Social and preventive pharmacy for Diploma and Bachelor of pharmacy
South African Journal of Science: Writing with integrity workshop (2024)

South African Journal of Science: Writing with integrity workshop (2024)Academy of Science of South Africa
A workshop hosted by the South African Journal of Science aimed at postgraduate students and early career researchers with little or no experience in writing and publishing journal articles.Pollock and Snow "DEIA in the Scholarly Landscape, Session One: Setting Expec...

Pollock and Snow "DEIA in the Scholarly Landscape, Session One: Setting Expec...National Information Standards Organization (NISO)
This presentation was provided by Steph Pollock of The American Psychological Association’s Journals Program, and Damita Snow, of The American Society of Civil Engineers (ASCE), for the initial session of NISO's 2024 Training Series "DEIA in the Scholarly Landscape." Session One: 'Setting Expectations: a DEIA Primer,' was held June 6, 2024.Recently uploaded (20)
Film vocab for eal 3 students: Australia the movie

Film vocab for eal 3 students: Australia the movie
Liberal Approach to the Study of Indian Politics.pdf

Liberal Approach to the Study of Indian Politics.pdf
The simplified electron and muon model, Oscillating Spacetime: The Foundation...

The simplified electron and muon model, Oscillating Spacetime: The Foundation...
How to Setup Warehouse & Location in Odoo 17 Inventory

How to Setup Warehouse & Location in Odoo 17 Inventory
Your Skill Boost Masterclass: Strategies for Effective Upskilling

Your Skill Boost Masterclass: Strategies for Effective Upskilling
Digital Artefact 1 - Tiny Home Environmental Design

Digital Artefact 1 - Tiny Home Environmental Design
How to Build a Module in Odoo 17 Using the Scaffold Method

How to Build a Module in Odoo 17 Using the Scaffold Method
LAND USE LAND COVER AND NDVI OF MIRZAPUR DISTRICT, UP

LAND USE LAND COVER AND NDVI OF MIRZAPUR DISTRICT, UP
Chapter 4 - Islamic Financial Institutions in Malaysia.pptx

Chapter 4 - Islamic Financial Institutions in Malaysia.pptx
Exploiting Artificial Intelligence for Empowering Researchers and Faculty, In...

Exploiting Artificial Intelligence for Empowering Researchers and Faculty, In...
Community pharmacy- Social and preventive pharmacy UNIT 5

Community pharmacy- Social and preventive pharmacy UNIT 5
South African Journal of Science: Writing with integrity workshop (2024)

South African Journal of Science: Writing with integrity workshop (2024)
Pollock and Snow "DEIA in the Scholarly Landscape, Session One: Setting Expec...

Pollock and Snow "DEIA in the Scholarly Landscape, Session One: Setting Expec...
Ch 05 Thermodynamics (1).ppt
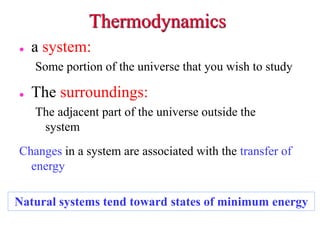
- 1. Thermodynamics a system: Some portion of the universe that you wish to study The surroundings: The adjacent part of the universe outside the system Changes in a system are associated with the transfer of energy Natural systems tend toward states of minimum energy
- 2. Energy States Unstable: falling or rolling Stable: at rest in lowest energy state Metastable: in low-energy perch Figure 5.1. Stability states. Winter (2001) An Introduction to Igneous and Metamorphic Petrology. Prentice Hall.
- 3. Gibbs Free Energy Gibbs free energy is a measure of chemical energy All chemical systems tend naturally toward states of minimum Gibbs free energy G = H - TS Where: G = Gibbs Free Energy H = Enthalpy (heat content) T = Temperature in Kelvins S = Entropy (can think of as randomness)
- 4. Thermodynamics a Phase: a mechanically separable portion of a system Mineral Liquid Vapor a Reaction: some change in the nature or types of phases in a system reactions are written in the form: reactants = products
- 5. Thermodynamics The change in some property, such as G for a reaction of the type: 2 A + 3 B = C + 4 D DG = S (n G)products - S(n G)reactants = GC + 4GD - 2GA - 3GB
- 6. Thermodynamics For a phase we can determine V, T, P, etc., but not G or H We can only determine changes in G or H as we change some other parameters of the system Example: measure DH for a reaction by calorimetry - the heat given off or absorbed as a reaction proceeds Arbitrary reference state and assign an equally arbitrary value of H to it: Choose 298.15 K and 0.1 MPa (lab conditions) ...and assign H = 0 for pure elements (in their natural state - gas, liquid, solid) at that reference
- 7. Thermodynamics In our calorimeter we can then determine DH for the reaction: Si (metal) + O2 (gas) = SiO2 DH = -910,648 J/mol = molar enthalpy of formation of quartz (at 298, 0.1) It serves quite well for a standard value of H for the phase Entropy has a more universal reference state: entropy of every substance = 0 at 0K, so we use that (and adjust for temperature) Then we can use G = H - TS to determine G of quartz = -856,288 J/mol
- 8. Thermodynamics For other temperatures and pressures we can use the equation: dG = VdP – SdT (ignoring DX for now) where V = volume and S = entropy (both molar) We can use this equation to calculate G for any phase at any T and P by integrating z z G G VdP SdT T P T P T T P P 2 1 1 1 2 1 2 2 - = -
- 9. Thermodynamics If V and S are constants, our equation reduces to: GT2 P2 - GT1 P1 = V(P2 - P1) - S (T2 - T1) which ain’t bad!
- 10. Thermodynamics In Worked Example 1 we used GT2 P2 - GT1 P1 = V(P2 - P1) - S (T2 - T1) and G298, 0.1 = -856,288 J/mol to calculate G for quartz at several temperatures and pressures Low quartz Eq. 1 SUPCRT P (MPa) T (C) G (J) eq. 1 G(J) V (cm3) S (J/K) 0.1 25 -856,288 -856,648 22.69 41.36 500 25 -844,946 -845,362 22.44 40.73 0.1 500 -875,982 -890,601 23.26 96.99 500 500 -864,640 -879,014 23.07 96.36 Agreement is quite good (< 2% for change of 500o and 500 MPa or 17 km)
- 11. Thermodynamics Summary thus far: G is a measure of relative chemical stability for a phase We can determine G for any phase by measuring H and S for the reaction creating the phase from the elements We can then determine G at any T and P mathematically Most accurate if know how V and S vary with P and T • dV/dP is the coefficient of isothermal compressibility • dS/dT is the heat capacity (Cp) Use? If we know G for various phases, we can determine which is most stable Why is melt more stable than solids at high T? Is diamond or graphite stable at 150 km depth? What will be the effect of increased P on melting?
- 12. Does the liquid or solid have the larger volume? High pressure favors low volume, so which phase should be stable at high P? Does liquid or solid have a higher entropy? High temperature favors randomness, so which phase should be stable at higher T? We can thus predict that the slope of solid-liquid equilibrium should be positive and that increased pressure raises the melting point. Figure 5.2. Schematic P-T phase diagram of a melting reaction. Winter (2001) An Introduction to Igneous and Metamorphic Petrology. Prentice Hall.
- 13. Does the liquid or solid have the lowest G at point A? What about at point B? The phase assemblage with the lowest G under a specific set of conditions is the most stable Figure 5-2. Schematic P-T phase diagram of a melting reaction. Winter (2001) An Introduction to Igneous and Metamorphic Petrology. Prentice Hall.
- 14. Free Energy vs. Temperature dG = VdP - SdT at constant pressure: dG/dT = -S Because S must be (+) G for a phase decreases as T increases Would the slope for the liquid be steeper or shallower than that for the solid? Figure 5.3. Relationship between Gibbs free energy and temperature for a solid at constant pressure. Teq is the equilibrium temperature. Winter (2001) An Introduction to Igneous and Metamorphic Petrology. Prentice Hall.
- 15. Free Energy vs. Temperature Slope of GLiq > Gsol since Ssolid < Sliquid A: Solid more stable than liquid (low T) B: Liquid more stable than solid (high T) Slope dP/dT = -S Slope S < Slope L Equilibrium at Teq GLiq = GSol Figure 5.3. Relationship between Gibbs free energy and temperature for the solid and liquid forms of a substance at constant pressure. Teq is the equilibrium temperature. Winter (2001) An Introduction to Igneous and Metamorphic Petrology. Prentice Hall.
- 16. Now consider a reaction, we can then use the equation: dDG = DVdP - DSdT (again ignoring DX) For a reaction of melting (like ice water) DV is the volume change involved in the reaction (Vwater - Vice) similarly DS and DG are the entropy and free energy changes dDG is then the change in DG as T and P are varied DG is (+) for S L at point A (GS < GL) DG is (-) for S L at point B (GS > GL) DG = 0 for S L at point x (GS = GL) DG for any reaction = 0 at equilibrium
- 17. Pick any two points on the equilibrium curve DG = ? at each Therefore dDG from point X to point Y = 0 - 0 = 0 dDG = 0 = DVdP - DSdT X Y dP dT DS = DV
- 18. Figures I don’t use in class Figure 5.4. Relationship between Gibbs free energy and pressure for the solid and liquid forms of a substance at constant temperature. Peq is the equilibrium pressure. Winter (2001) An Introduction to Igneous and Metamorphic Petrology. Prentice Hall.
- 19. Figures I don’t use in class Figure 5.5. Piston-and-cylinder apparatus to compress a gas. Winter (2001) An Introduction to Igneous and Metamorphic Petrology. Prentice Hall.
Editor's Notes
- a system: Some portion of the universe that you wish to study a glass of water plagioclase the mantle Changes in a system are associated with the transfer of energy lift an object: stored chemical potential drop an object: potential kinetic pump up a bicycle tire: chemical mechanical heat (friction + compression) add an acid to a base: chemical heat
- The side of the reaction with lower G will be more stable How do we go about determining this for a reaction? First we must be able to determine G for the phases in the reaction at any P and T
- To integrate properly we must know how V and S vary with P and T (hence the calculus), but we shall simplify the math and assume V and S are constant This simplifies our math considerably (but may lead to some errors) We can check the validity of our assumptions by comparing to experiments or more rigorous calculations (performed by computer)
- The P and T corrections can be done separately in any order, since we are concerned only with the initial and final states, not the path the system followed to get from one to the other
- All of these questions depend on reactions comparing 2 or more phases
- Consider the phase diagram (as we anticipate igneous petology) We can apply thermodynamics qualitatively to assess these diagrams
- From dG = VdP - SdT we can deduce that at constant pressure: dG = -SdT And since S must be (+) G for a phase decreases as T increases