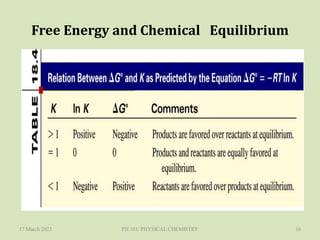
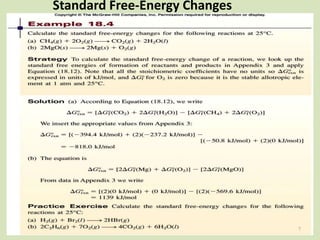
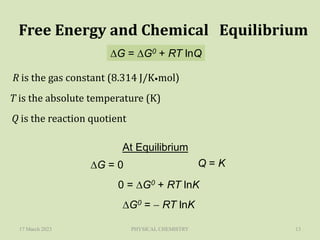
The document discusses Gibbs free energy and its relationship to chemical equilibrium. It defines Gibbs free energy and how it can be used to predict the spontaneity of reactions. The Gibbs free energy change, ΔG, is related to the standard Gibbs free energy change, ΔG°, and the reaction quotient, Q, by the equation ΔG = ΔG° + RTlnQ. At equilibrium when ΔG = 0, Q equals the equilibrium constant, K. Expressions are provided for calculating K for different types of reactions, such as gas phase, liquid phase, and liquid-solid reactions. The key points are that a reaction will only proceed spontaneously if ΔG is negative and that a reaction will reach equilibrium when ΔG equals




![10
• For a chemical reaction vAA + vBB D vCC + vDD
when G°T =0, we say the reaction is in chemical reaction equilibrium
- What is a chemical reaction equilibrium?
Example 1: NH3(aq)+H2O(l) D NH4
+(l)+OH-(aq)
At constant T and P, when t
Example 2: 2NO(g) + O2(g) D 2NO2(g)
At constant T and P, when t
The concentration of a gas is usually measured as partial pressure
At an equilibrium the reaction quotient becomes constant
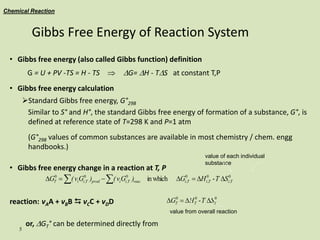
Chemical Reaction - The Equilibrium
t
NO2
NO
O2
t
NH4
NH3
constant
2
2
2
2
O
NO
NO
P
P
P
constant
O]
][H
[NH
]
][OH
[NH
2
3
4
reaction quotient](https://image.slidesharecdn.com/4pchlecture-230317041429-c0a5da14/85/4-PCh-Lecture-ppt-10-320.jpg)
![11
Equilibrium Constant (1)
• Definition of equilibrium constant, K
The equilibrium constant is the reaction quotient at G°T =0.
• Expressions of equilibrium constant for various reactions
– gas phase 2NO(g) + O2(g) D 2NO2(g)
– gas-solid phase CaCO3(s) D CaO (s)+CO2(g)
– liquid phase NH3(aq)+H2O(l) D NH4
+(l)+OH-(aq)
– liquid-solid Cu(OH)2(s) D Cu2+(aq)+2OH- (aq)
– gas-liquid NH3(g)+H2O(l) D NH4OH(aq)
– general vAA + vBB D vCC + vDD
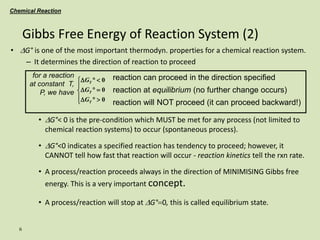
Chemical Reaction equilibrium
2
2
2
2
O
NO
NO
p
P
P
P
K
O]
][H
[NH
]
][OH
[NH
2
3
4
c
K
2
CO
p P
K
2
2
]
][OH
[Cu
c
K
3
1 NH
p P
/
K
B
A
D
C
B
A
D
C
v
B
v
A
v
D
v
C
v
v
v
v
c
P
P
P
P
D
C
K
[B]
[A]
]
[
]
[](https://image.slidesharecdn.com/4pchlecture-230317041429-c0a5da14/85/4-PCh-Lecture-ppt-11-320.jpg)
![12
• Equilibrium constant, Kp, and Gibbs free energy DG
A gas phase reaction vAA + vBB vCC + vDD
– When at equilibrium, assuming all the gases follow the ideal gas law
at P=1 atm, DG°=S(viG°i)prod-S(viG°i)reac=0
and at any P(1) DG=S(viGi)prod-S(viGi)reac=0
– when reaction occurs at constant temperature (isothermal reaction)
dG=VdP-SdT dG=VdP dG=RTdP/P DG=RTln(P/P0)
– G° is defined at P0=1 atm, so that DG=G-G°=RTln(P/1) Gi=Gi°+RTlnPi
S(vi(G°i+RTln(PCPD))prod-S(vi (G°i+RTln(PAPB))reac=0
S(viG°i)prod-S(vi G°i)reac=-[S(viRTln(PCPD))prod-S(vi RTln(PAPB))reac]
– By definition:
Equilibrium Constant (2)
Chemical Reaction equilibrium
D B
A
D
C
B
A
D
C
v
B
v
A
v
D
v
C
v
B
v
A
v
D
v
C
P
P
P
P
ln
RT
)
atm
/
P
(
)
atm
/
P
(
)
atm
/
P
(
)
atm
/
P
(
ln
RT
G
p
K
RT
G ln
D
B
A
D
C
v
B
v
A
v
D
v
C
p
P
P
P
P
K
T=const PV=RT intergration](https://image.slidesharecdn.com/4pchlecture-230317041429-c0a5da14/85/4-PCh-Lecture-ppt-12-320.jpg)
![14
• Equilibrium constant, Kc, and Gibbs free energy DG
– Ideal solution (liquid and solid)
Raoult’s law Pi=xiPi* or xi=Pi / Pi* where
thus for a solution we can also write Gi=(Gi°+RTln xi) (compare to gas Gi=Gi°+RTlnPi)
which lead to, in a similar way, the relation between DG and Kc,
• Summary of G calculation for ideal gas and solution
– For a pure substance at const T & P, G=G°+RTlnP (1)
– For a mixture of ideal gas at const T and P G =SGi=S(Gi°+RTln Pi) (2-1)
– For a mixture of ideal solution at const T and P G =SGi=S(Gi°+RTln xi) (2-2)
For a situation that a mixture (gas or solution) under concern is not ideal, the Pi or xi cannot be
related to G by expressions (2-1) & (2-2). How do you calculate G?
Equilibrium Constant (3)
Chemical Reaction equilibrium
Pi - vapour pressure of component i
xi - mole fraction of component i in solution
Pi* - equil. vapour pressure of pure component i
B
A
D
C
B
A
D
C
B
A
D
C
v
v
v
v
v
v
v
v
v
B
v
A
v
D
v
C
K
K
RT
RT
x
x
x
x
RT
G
[B]
[A]
[D]
[C]
where
)
ln(
[B]
[A]
[D]
[C]
ln
ln c
c
D](https://image.slidesharecdn.com/4pchlecture-230317041429-c0a5da14/85/4-PCh-Lecture-ppt-14-320.jpg)