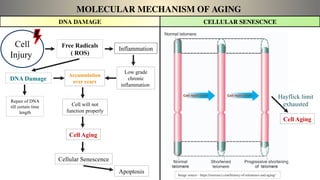
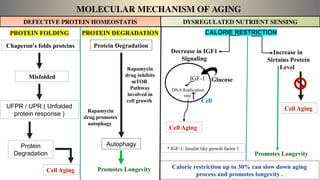
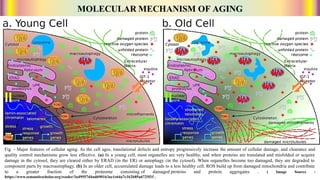
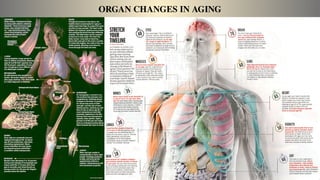
The document discusses the complex phenomenon of aging, outlining various theories that explain the mechanisms behind cellular aging, such as programmed aging, oxidative stress, and telomere shortening. It highlights the impact of genetics, environmental factors, and lifestyle choices on aging while suggesting strategies like caloric restriction and pharmacological interventions that may help extend lifespan and healthspan. Emerging technologies, including gene editing and cellular reprogramming, present new possibilities for revitalizing aged cells and improving health outcomes.
![M.Sc. Zoology Batch : 2022-2024
S.S KHANNA GIRLS DEGREE COLLEGE
[ Constituent College Of University Of Allahabad ]](https://image.slidesharecdn.com/cellagingishitabhardwajm-241226112353-b94c884b/75/Cellular-Aging-and-Its-Molecular-Mechanism-1-2048.jpg)