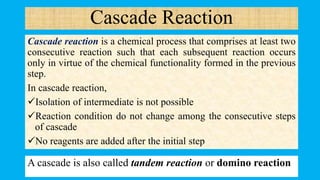
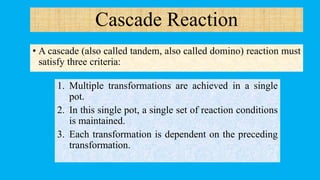
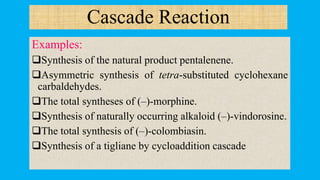
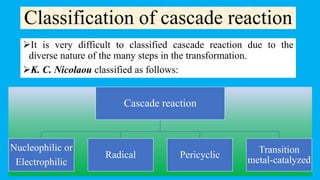
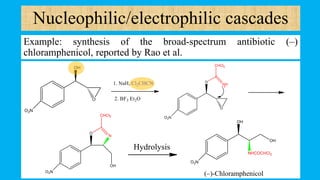
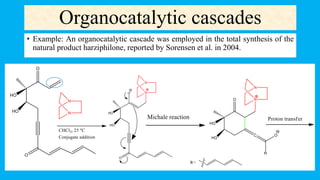
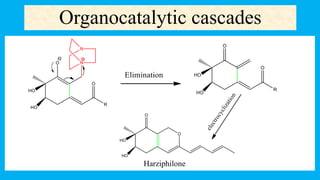
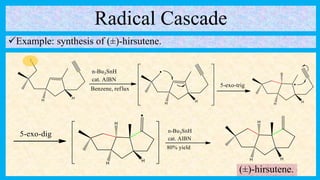
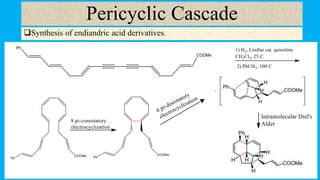
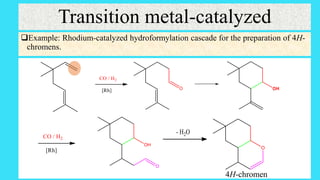
This document defines and provides examples of cascade reactions. A cascade reaction involves at least two consecutive reactions where the product of one reaction undergoes the next without isolation of intermediates or changing conditions. Cascade reactions are classified as nucleophilic/electrophilic, radical, pericyclic, or transition metal-catalyzed. Examples provided include syntheses of natural products like pentalenene and morphine using these cascade reaction types.