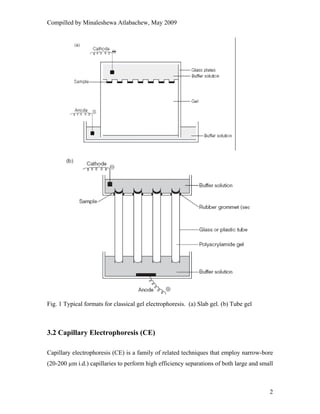
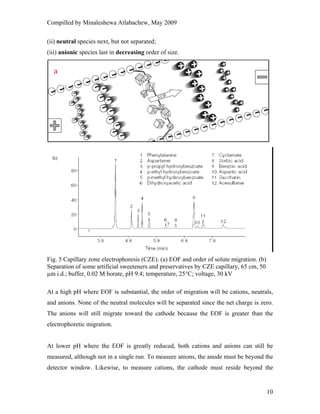
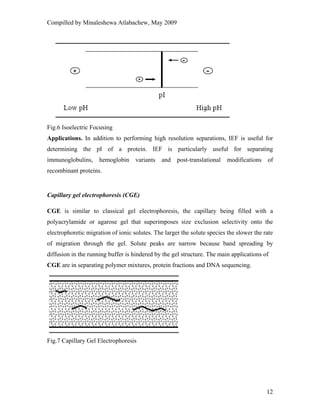
This document provides an overview of capillary electrophoresis. It defines key terminology used in capillary electrophoresis such as migration time, electrophoretic mobility, electroosmotic flow, efficiency, and resolution. It also describes the main modes of capillary electrophoresis including capillary zone electrophoresis, isoelectric focusing, capillary gel electrophoresis, and micellar electrokinetic capillary chromatography. For each mode, it provides a brief explanation of how separation is achieved. The document concludes by noting that capillary electrophoresis techniques can be used to separate a variety of analytes including proteins, peptides, inorganic ions, and pharmaceutical compounds.