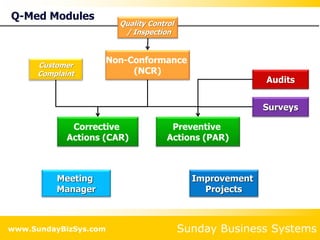
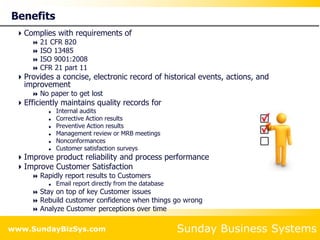
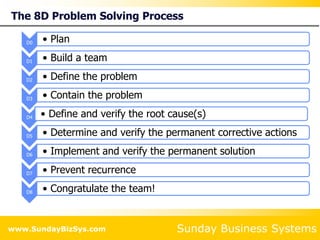
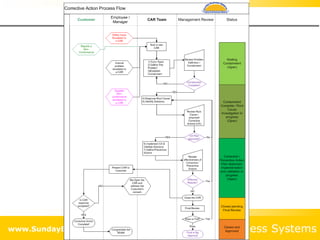
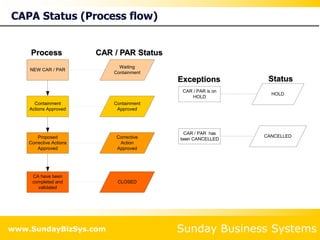
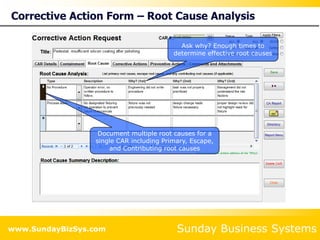
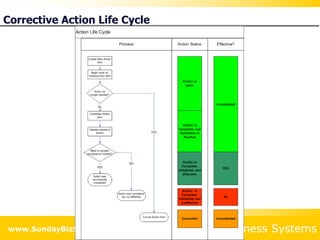
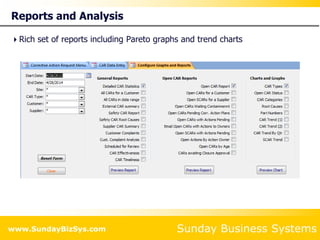
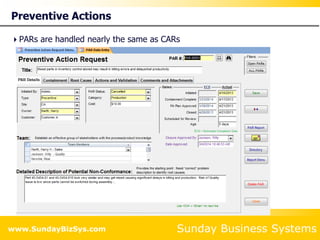
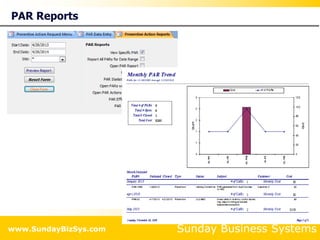
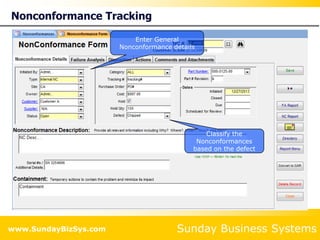
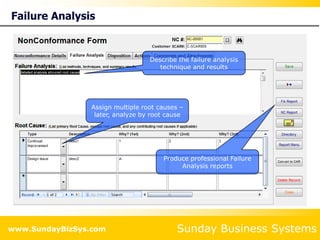
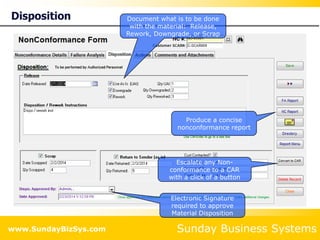
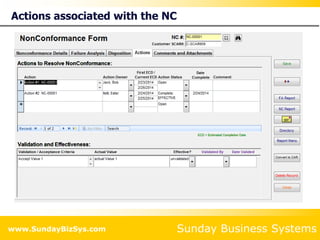
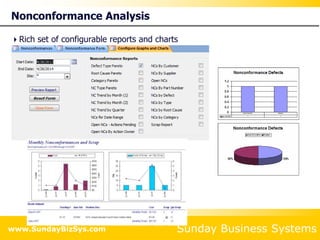
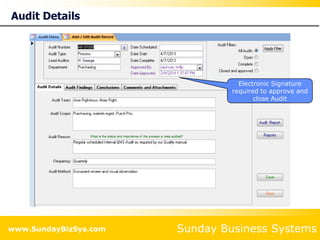
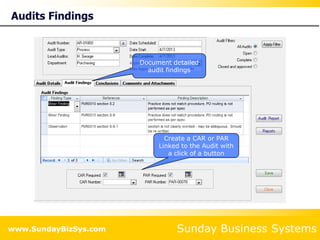
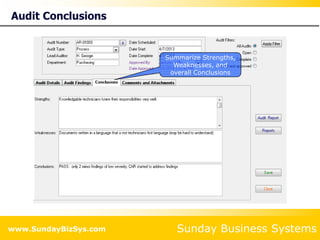
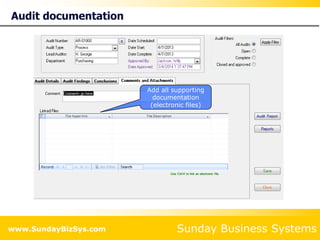
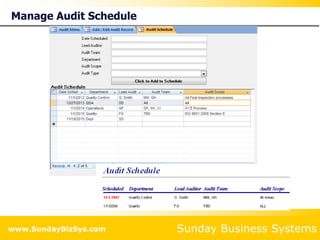
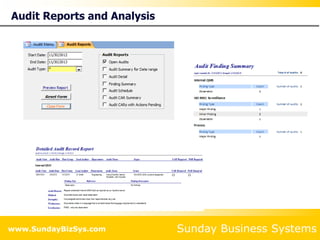
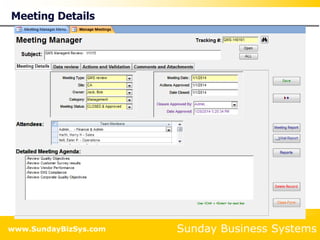
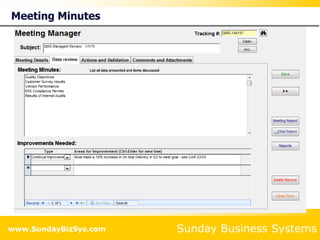
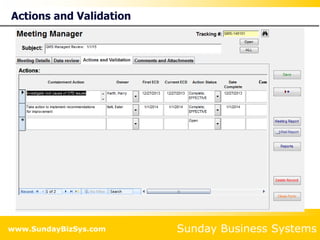
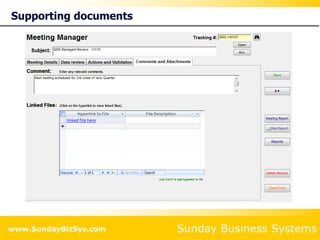
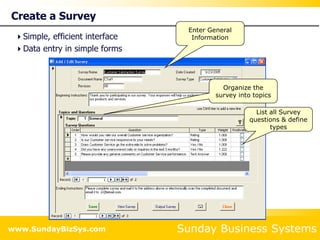
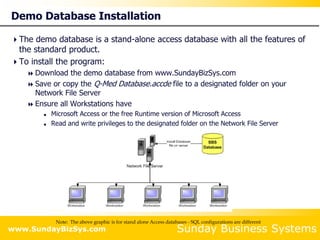
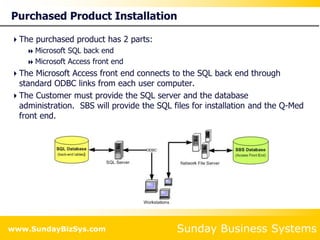
This document summarizes the features and benefits of the SBS Q-Med Database software for managing quality systems in medical device and FDA-regulated industries. The software allows users to track nonconformances, corrective and preventive actions, audits, meetings, and customer satisfaction surveys. It provides templates for documentation, electronic signatures, and reporting functionality to analyze data. The software is intended to help users comply with quality standards like ISO 13485, 21 CFR 820 and improve processes through effective management of quality records and metrics.