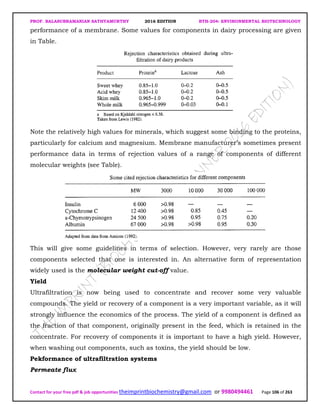
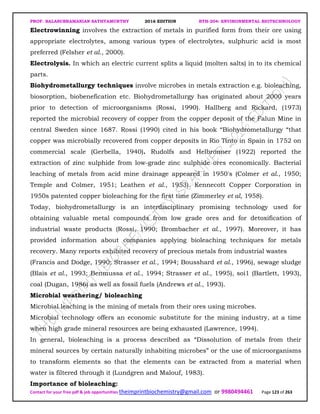
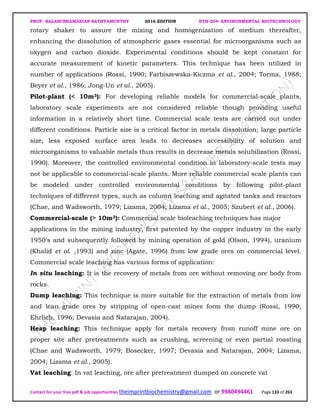
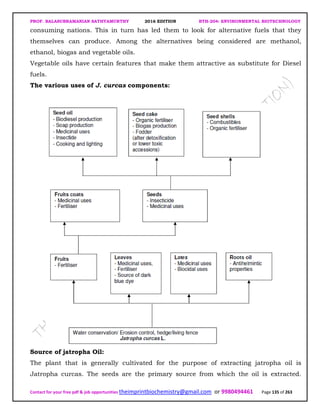
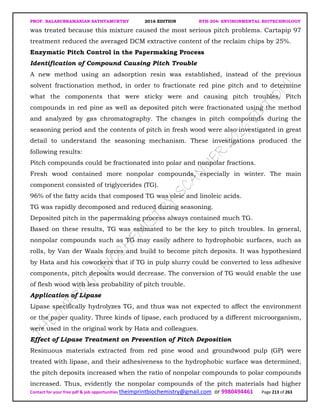
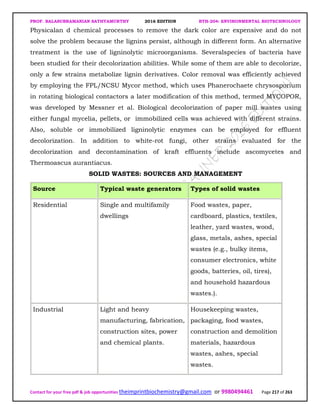
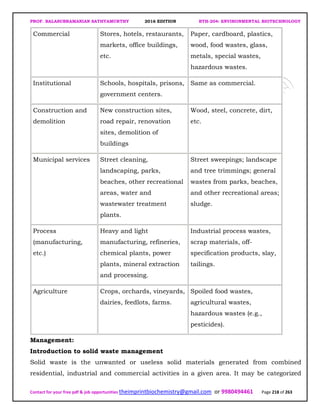
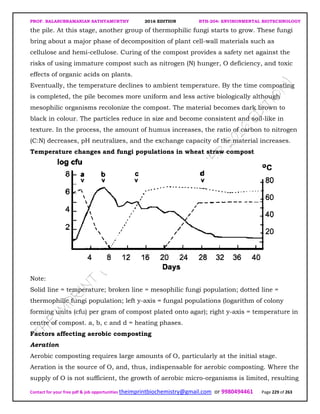
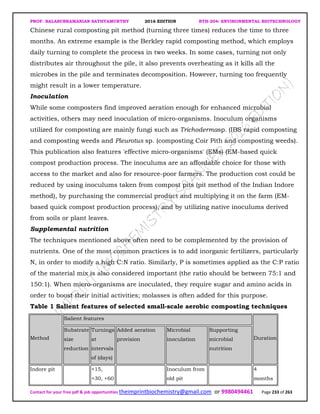
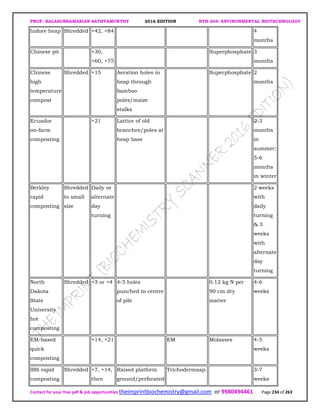
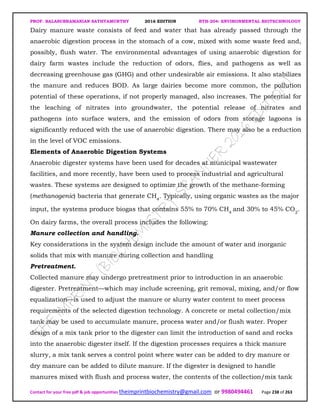
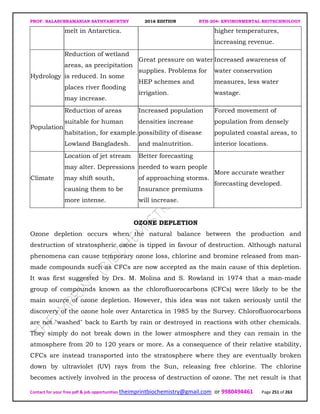
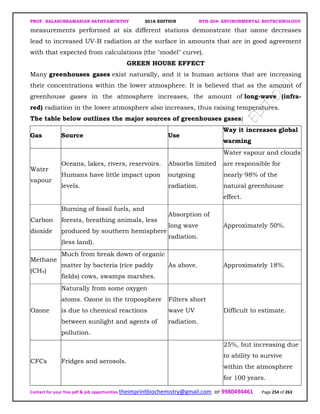
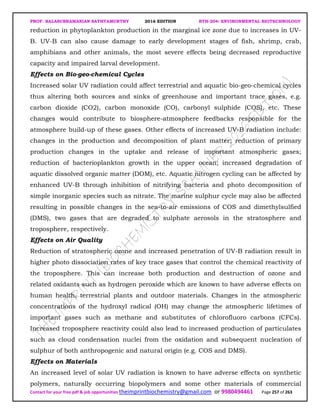
The document discusses environmental biotechnology, which deals with topics like cleaning up contamination and managing wastes. It has relevance for many industries as it can help reduce costs and improve compliance with environmental regulations. Some key applications of environmental biotechnology include waste treatment, pollution control during manufacturing, and bioremediation of contaminated land. It provides alternatives to traditional waste disposal and chemical-based cleanup methods that are more sustainable and cost-effective. The economic factors will always influence the adoption of environmental biotechnology solutions.









































![PROF. BALASUBRAMANIAN SATHYAMURTHY 2016 EDITION BTH-204: ENVIRONMENTAL BIOTECHNOLOGY
Contact for your free pdf & job opportunities theimprintbiochemistry@gmail.com or 9980494461 Page 44 of 263
environmental contamination or as bioremediation process monitoring and control tools
to provide informational data on what contaminants are present, where they are
located, and a very sensitive and accurate evaluation of their concentrations in terms of
bioavailability. Bioavailability measurements are central to environmental monitoring as
well as risk assessment because they indicate the biological effect of the chemical,
whether toxic, cytotoxic, genotoxic, mutagenic, carcinogenic, or endocrine disrupting,
rather than mere chemical presence as is achieved with analytical instruments.
Despite their benefits, biosensors remain relatively unused in the environmental
monitoring/bioremediation fields, due primarily to a lack of real-world, real-sample
testing and standardization against conventional analytical techniques. Thus, although
biosensors show significant promise, it is clear that more field validation studies need to
be performed before regulatory agencies and other end users will gain sufficient
confidence to adopt their routine use.
Enzyme-based biosensors
The historical foundation of the biosensor rests with the enzyme glucose oxidase and its
immobilization on an oxygen electrode by Leland Clark in the 1960s for blood glucose
sensing. Although the research emphasis of enzyme-based biosensors continues to be
driven by more lucrative medical diagnostics, there has been a predictable application
overlap toward environmental monitoring as well. Enzymes act as organic catalysts,
mediating the reactions that convert substrate into product. Since enzymes are highly
specific for their particular substrate, the simplest and most selective enzyme-based
biosensors merely monitor enzyme activity directly in the presence of the substrate.
Perhaps the most relevant examples are the sulfur/sulfate-reducing bacterial
cytochrome c3 reductases that reduce heavy metals. Michel et al. (2003) immobilized
cytochrome c3 on a glassy carbon electrode and monitored its redox activity
amperometrically in the presence of chromate [Cr(VI)] with fair sensitivity (lower
detection limit of 0.2 mg/L) and rapid response (several minutes) (Figure 9.2). When
tested under simulated groundwater conditions, the biosensor did cross-react with
several other metal species, albeit at lower sensitivities, and was affected by
environmental variables such as pH, temperature, and dissolved oxygen, thus
exemplifying certain disadvantages common to enzyme-based biosensors. Similarly
operated biosensors for the groundwater contaminant perchlorate using perchlorate
reductase as the recognition enzyme (detection limit of 10 µg/L) (Okeke et al., 2007),](https://image.slidesharecdn.com/bth204environmentalbiotechnology-190404171749/85/Bth-204-environmental-biotechnology-44-320.jpg)
![PROF. BALASUBRAMANIAN SATHYAMURTHY 2016 EDITION BTH-204: ENVIRONMENTAL BIOTECHNOLOGY
Contact for your free pdf & job opportunities theimprintbiochemistry@gmail.com or 9980494461 Page 45 of 263
organophosphate pesticides using parathion hydrolase or organophosphorus hydrolase
as recognition enzymes (detection down to low µM concentrations) (Trojanowicz, 2003),
and environmental estrogens using tyrosinase as the recognition enzyme (detection
down to 1 µM) (Andreescu and Sadik, 2004) have also been designed.
Another type of enzyme biosensor relies on enzyme activation upon interaction with the
target of interest. For example, heavy metals in the form of cofactors—inorganic ions
that bind to and activate the enzyme—can be detected based on this integral
association.
Metalloenzymes such as alkaline phosphatase, ascorbate oxidase, glutamine
synthetase, and carbonic anhydrase require association of a metal ion cofactor with
their active sites for catalytic activity, and can thus be used as recognition elements for
heavy metals. Strong chelating agents are first used to strip the enzyme of all metal ion
cofactors to form the inactive apoenzyme. Upon exposure to the sample, the apoenzyme
binds any metal ions present and is reactivated, and this rate of reactivation can be
related directly to the stoichiometric amount of metal complexed to the enzyme’s active
site. Alkaline phosphatase, for example, can be applied in this regard as a biosensor for
zinc [Zn(II)] or ascorbate oxidase for biosensing copper(II) with detection limits down to
very low part-per-billion levels (Satoh and Iijima, 1995).
However, as various other metals as well as other sample cross-contaminants can act
as cofactors and/or inhibitors of the metalloenzyme, selectivity becomes somewhat
problematic. To enhance selectivity, molecular techniques such as site-directed
mutagenesis or directed evolution can be used to genetically engineer or select for
enzymes with superior specificity for the target, as has been accomplished with
carbonic anhydrase and its selective biosensing of Zn(II) (Fierke and Thompson,
2001).Alternatively, and more commonly, target analytes such as heavy metals can also
inhibit enzyme activity, thereby diminishing the conversion of substrate to product. By
monitoring subsequent Michaelis–Menten rate kinetics, a highly sensitive measurement
of target can be obtained, often at picomolar detection limits, with little prerequisite
sample processing. However, selectivity cannot be ascertained since the specificity of
enzymes toward inhibitors is not target specific. Thus, inhibitor-based biosensors detect
the global presence of heavy metals rather than identifying a particular heavy metal ion
in a sample. The standard suite of enzymes used in these biosensors includes oxidases,
urease, alkaline phosphatase, choline esterases, and invertase for the detection of](https://image.slidesharecdn.com/bth204environmentalbiotechnology-190404171749/85/Bth-204-environmental-biotechnology-45-320.jpg)


![PROF. BALASUBRAMANIAN SATHYAMURTHY 2016 EDITION BTH-204: ENVIRONMENTAL BIOTECHNOLOGY
Contact for your free pdf & job opportunities theimprintbiochemistry@gmail.com or 9980494461 Page 48 of 263
single contaminant. There are numerous examples of bioreporters that detect toxicity,
most of which contain a reporter gene fused to a strong constitutive promoter such that
a decrease in signal indicates a toxic response. Bhattacharyya et al. (2005) utilized two
such bacterial bioreporters (bioluminescent Pseudomonas putida and Escherichia coli ),
along with a naturally bioluminescent Vibrio fischeri (recently reclassified as Aliivibrio
fischeri ), to identify areas of a contaminated groundwater site in southern England that
contained toxic substances. They also used two “catabolic” bioreporter strains for
monitoring trichloroethylene (TCE) and compared data from all of these strains to
chemical data, with the goal of gathering data to assess the bioavailability of
contaminants in the groundwater.
The three bacterial toxicity bioreporters differed in their assessment of the toxic
potential for the samples, attributed to each strain being more or less sensitive to the
primary pollutants in each sample, underscoring the benefit of using multiple
bioreporters within biosensors.
Antibody-based biosensors (Immunosensors)
Biosensors that use antibodies as recognition elements (immunosensors) are used
widely as environmental monitors because antibodies are highly specific, versatile, and
bind stably and strongly to target analytes (antigens). This high affinity for target,
however, can also be disadvantageous since the target cannot easily be released from
the antibody after the measurement has been made, resulting in many antibody-based
biosensors being single-use disposable units [although release (regeneration) can be
promoted by the addition of organic solvents or chaotrophic reagents, this requirement
for a supplementary assay step is not optimal]. Additionally, the synthesis of antibodies
and further testing and optimization of their target affinities can be a long, tedious, and
expensive process, cross-reactivity with multiple analytes can occur, and
antibody/antigen reactivity can be affected by environmental variables such as pH and
temperature (see Hock, 2000 for a review). Nonetheless, antibodies can be highly
effective detectors for environmental contaminants, and advancements in techniques
such as phage display for the preparation and selection of recombinant antibodies with
novel binding properties assures their continued environmental application. Perhaps
the best introduction to antibody-based biosensing is the AWACSS (Automated Water
Analyzer Computer Supported System) environmental monitoring system developed for
remote, unattended, and continuous detection of organic pollutants for water quality](https://image.slidesharecdn.com/bth204environmentalbiotechnology-190404171749/85/Bth-204-environmental-biotechnology-48-320.jpg)
![PROF. BALASUBRAMANIAN SATHYAMURTHY 2016 EDITION BTH-204: ENVIRONMENTAL BIOTECHNOLOGY
Contact for your free pdf & job opportunities theimprintbiochemistry@gmail.com or 9980494461 Page 49 of 263
control [and also its predecessor, the RIANA (River Analyzer)] (Figure 9.6) (Tschmelak et
al., 2005).
AWACSS uses an optical evanescent wave transducer and fluorescently labeled
polyclonal antibodies for multiplexed detection of targeted groups of contaminants,
including endocrine disruptors, pesticides, industrial chemicals, pharmaceuticals, and
other priority pollutants, without requisite sample preprocessing. Antibody binding to a
target sample analyte occurs in a short 5-minute preincubation step, followed by
microfluidic pumping of the sample over the transducer element, which consists of an
optical waveguide chip impregnated with 32 separate wells of immobilized antigen
derivatives. As the antibody/analyte complexes flow through these wells, only
antibodies with free binding sites can attach to the well surface (in what is referred to
as a binding inhibition assay). Thus, antibodies with both of their binding sites bound
with analyte will not attach to the surface and will pass through the detector. A
semiconductor laser then excites the fluorophore label of bound antibodies, allowing for
their quantification, with high fluorescence signals indicating low analyte
concentrations and low fluorescence signals indicating high analyte concentrations. A
fiber optic array tied to each well permits separation and identification of signals by the
well, thereby yielding a simultaneous measurement of up to 32 different sample
contaminants.
The instrument has been used for groundwater, wastewater, surface water, and
sediment sample testing with detection limits for most analytes in the ng/L range
within assay times of approximately 18 minutes.
DNA-Based biosensors
The foundation of nucleic acid–based biosensors relies on a transducer capable of
monitoring a change in the nucleic acid’s structure occurring after exposure to a target
chemical. These structural changes are brought on either by the mutagenic nature of
the chemical, resulting in mutations, intercalations, and/or strand breaks, or by the
chemical’s ability to covalently or noncovalently attach to the nucleic acid. Immobilizing
the nucleic acid as a recognition layer on the transducer surface forms the biosensor,
and detection of the chemically induced nucleic acid conformational change is then
typically achieved electrochemically (i.e., a change in the current) or less so through
optical or other means (see Fojta, 2002 for an excellent review).](https://image.slidesharecdn.com/bth204environmentalbiotechnology-190404171749/85/Bth-204-environmental-biotechnology-49-320.jpg)















































































































































![PROF. BALASUBRAMANIAN SATHYAMURTHY 2016 EDITION BTH-204: ENVIRONMENTAL BIOTECHNOLOGY
Contact for your free pdf & job opportunities theimprintbiochemistry@gmail.com or 9980494461 Page 211 of 263
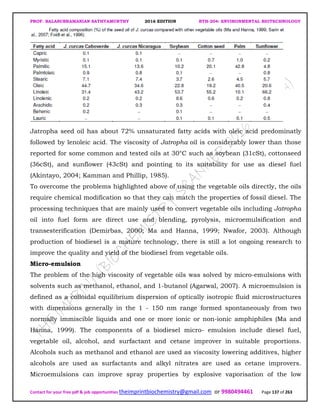
kilogram of the powder can treat about 1200 tons of wood chips. Industrial use involves
dispersing the powder in mill water and spraying it onto chips as they are conveyed to a
chip pile.
Selective breeding was used to obtain this isolate, which rapidly colonizes nonsterile
wood chips, rapidly degrades extractives, and is colorless and nonstaining. Most O.
piliferum strains are a bluish-black color. Growth of pigmented fungi on wood chips
reduces chip brightness and increases bleach usage when these chips are used to
produce TMP or sulfite pulp. Because Cartapip outcompetes indigenous
microorganisms and maintains chip brightness, use of this product reduces bleach
chemical usage during TMP production, in addition to reducing the extractive content of
chips and pulp, and alleviating pitch problems.
Use of treated chips has also been shown to increase paper strength. Moreover,
treatment of wood chips with Cartapip also results in improved chemical pulping
efficiency. Reductions in kappa number were observed during laboratory-scale kraft
and sulfite pulping. Wall et al. hypothesize that the improved pulping efficiency
observed experimentally is caused by more rapid and more uniform penetration of
steam and cooking chemicals in the fungally treated chips.
Two commercially available strains of O. piliferum, Cartapip 28 and Cartapip 58, have
been shown to degrade the extractives of both hardwoods and softwoods including
aspen, southern yellow pine, red pine, and spruce, Both fungi in two weeks reduced the
diethyl ether extractive content of fresh nonsterile southern yellow pine wood chips by
40%, or 22% if the chips were aged but not inoculated. In addition, the white strain,
Cartapip 58, maintained chip brightness. This study also showed that Cartapip 28
decreased the DCM extractive content of sterile southern yellow pine chips by 30% for a
2 week treatment. O. piliferum has also been shown to reduce more DCM extractives of
nonsterile red pine in 31 days (48% reduction) than in 21 days (32% reduction). O.
piliferum, strain Cartapip 97, also reduces the DCM extractives of spruce by 25% in a 2
week treatment, and the DCM extractive content of sterile loblolly pine chips by up to
35% in a 4 week treatment. Cartapip 97 reduced the acetone extractive content of fresh
nonsterile aspen chips by 36% after a 3 week incubation, or 13% for uninoculated aged
chips. Cartapip 28 and Cartapip 58 significantly decreased the fatty acid content and
the unidentified compound content, which includes waxes, alcohols, and sterols, of
southern yellow pine extractives l-6]. In particular, Cartapip 58 decreased esterified](https://image.slidesharecdn.com/bth204environmentalbiotechnology-190404171749/85/Bth-204-environmental-biotechnology-211-320.jpg)







































![PROF. BALASUBRAMANIAN SATHYAMURTHY 2016 EDITION BTH-204: ENVIRONMENTAL BIOTECHNOLOGY
Contact for your free pdf & job opportunities theimprintbiochemistry@gmail.com or 9980494461 Page 262 of 263
the conservation status of species is employed extensively in conservation
management, some scientists highlight that it is the common species that are the
primary source of exploitation and habitat alteration by humanity. Moreover, common
species are often undervalued despite their role as the primary source of ecosystem
services.
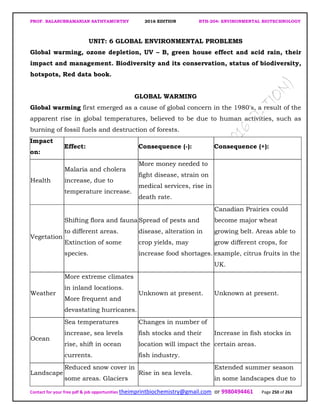
HOTSPOTS - RED DATA BOOK
While most in the community of conservation science "stress the importance"
of sustaining biodiversity, there is debate on how to prioritize genes, species, or
ecosystems, which are all components of biodiversity (e.g. Bowen, 1999). While the
predominant approach to date has been to focus efforts on endangered species by
conserving biodiversity hotspots, some scientists (e.g) and conservation organizations,
such as the Nature Conservancy, argue that it is more cost effective, logical, and
socially relevant to invest in biodiversity coldspots. The costs of discovering, naming,
and mapping out the distribution every species, they argue, is an ill advised
conservation venture. They reason it is better to understand the significance of the
ecological roles of species.
Biodiversity hotspots and coldspots are a way of recognizing that the spatial
concentration of genes, species, and ecosystems is not uniformly distributed on the
Earth's surface. For example, "[...] 44% of all species of vascular plants and 35% of all
species in four vertebrate groups are confined to 25 hotspots comprising only 1.4% of
the land surface of the Earth."
Those arguing in favor of setting priorities for coldspots point out that there are other
measures to consider beyond biodiversity. They point out that emphasizing hotspots
downplays the importance of the social and ecological connections to vast areas of the
Earth's ecosystems where biomass, not biodiversity, reigns supreme. It is estimated
that 36% of the Earth's surface, encompassing 38.9% of the world’s vertebrates, lacks
the endemic species to qualify as biodiversity hotspot. Moreover, measures show that
maximizing protections for biodiversity does not capture ecosystem services any better
than targeting randomly chosen regions. Population level biodiversity (i.e. coldspots) are
disappearing at a rate that is ten times that at the species level. The level of importance
in addressing biomass versus endemism as a concern for conservation biology is
highlighted in literature measuring the level of threat to global ecosystem carbon stocks
that do not necessarily reside in areas of endemism. A hotspot priority approach would](https://image.slidesharecdn.com/bth204environmentalbiotechnology-190404171749/85/Bth-204-environmental-biotechnology-262-320.jpg)
