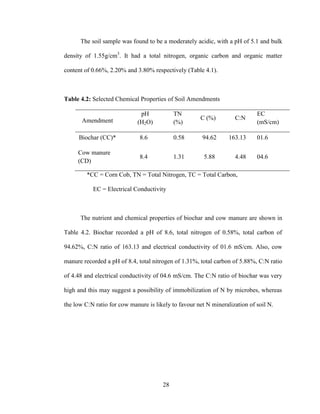
This document presents a dissertation written by Stephen Owusu that investigates the effect of rates of biochar additions on soil nitrogen dynamics and yield of lettuce in a tropical soil amended with cow manure. The study characterized biochar and soil properties, conducted an incubation experiment with biochar and cow manure treatments, and measured their effects on soil nitrogen concentrations and lettuce yield over time. The results showed that biochar and cow manure additions significantly increased soil organic carbon, pH, and lettuce yield compared to the control. Biochar additions resulted in higher nitrate concentrations while cow manure led to higher ammonium. It is recommended that farmers use a combination of biochar and cow manure to maximize yields and soil properties.