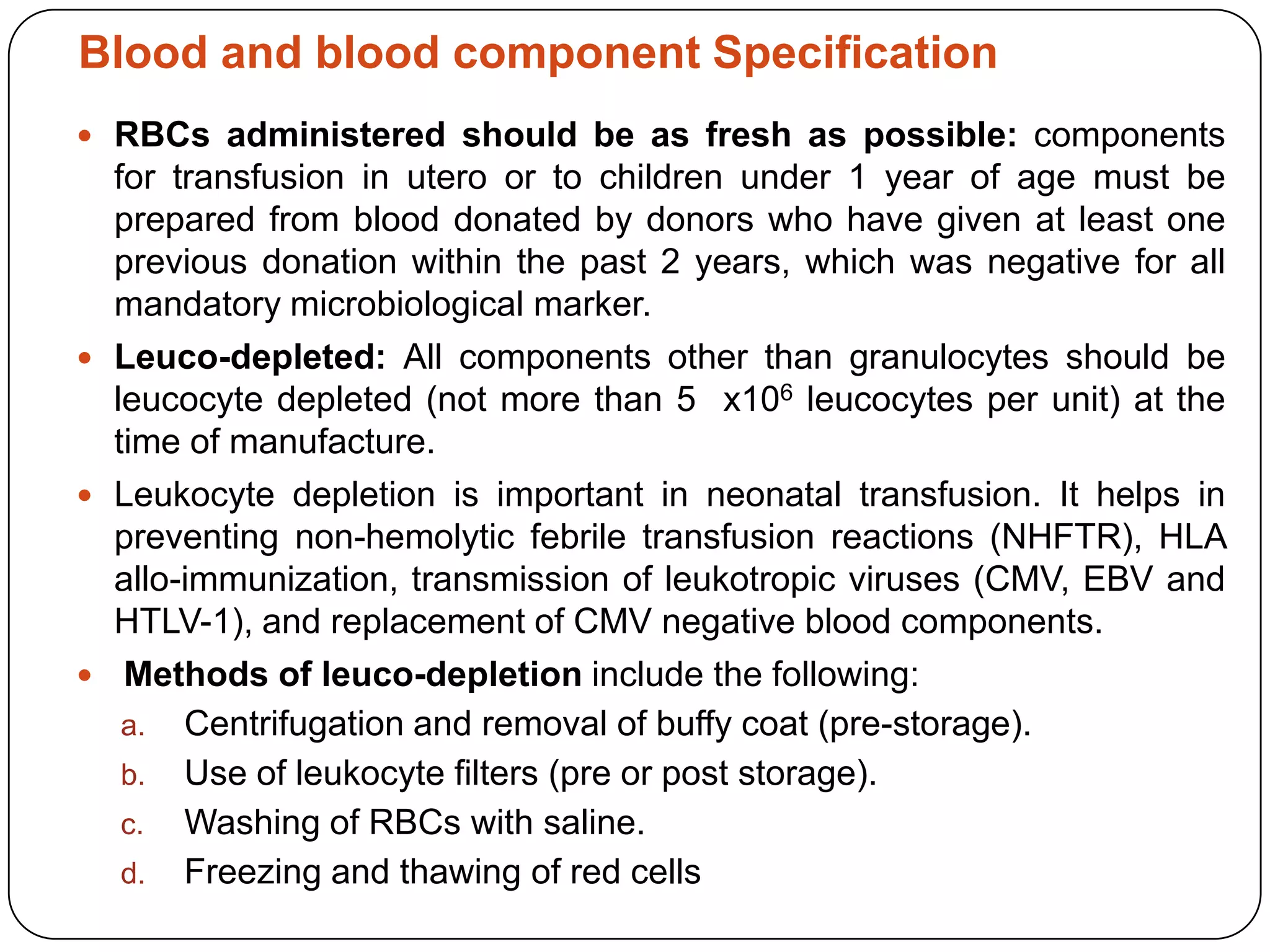
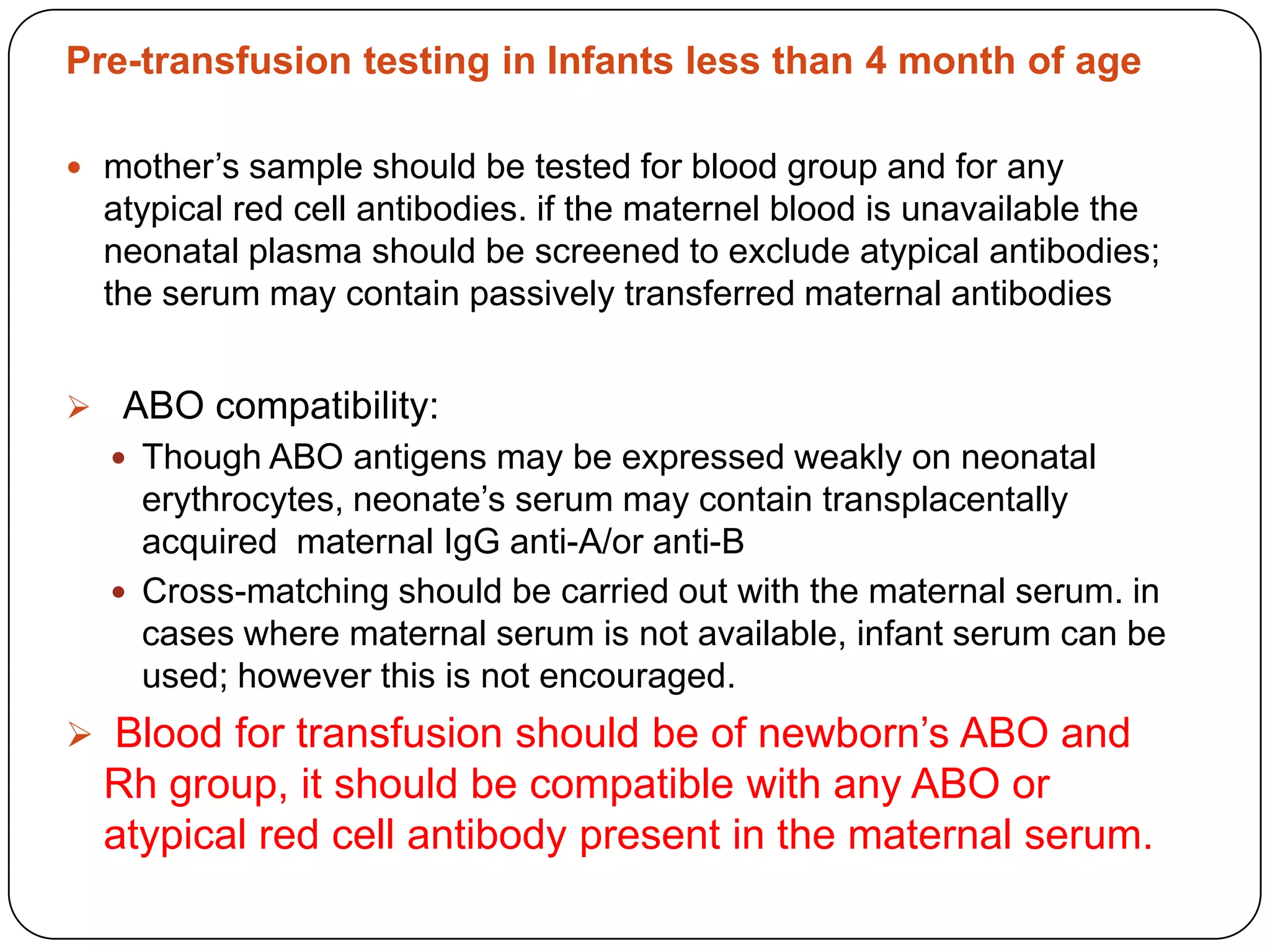
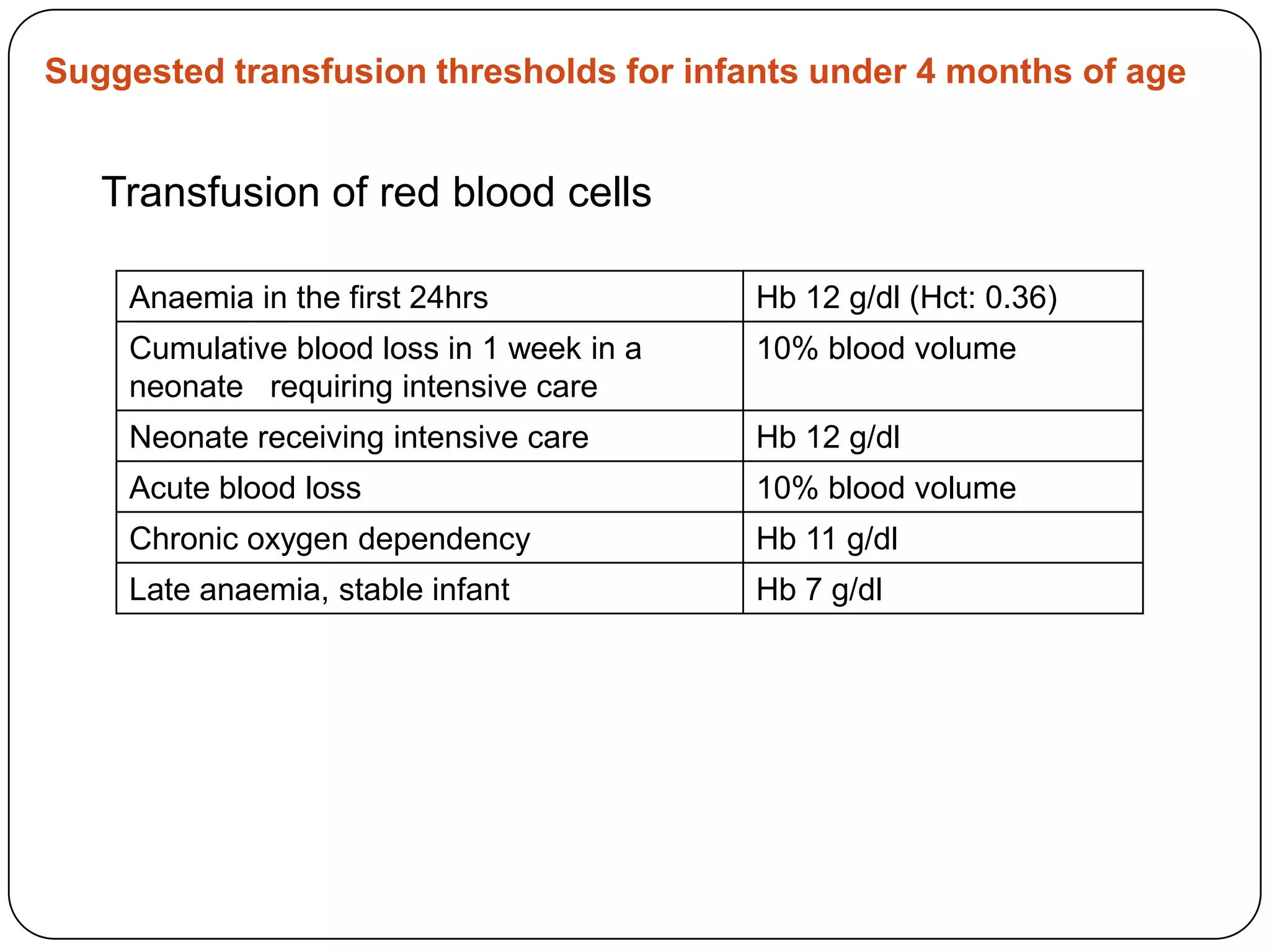
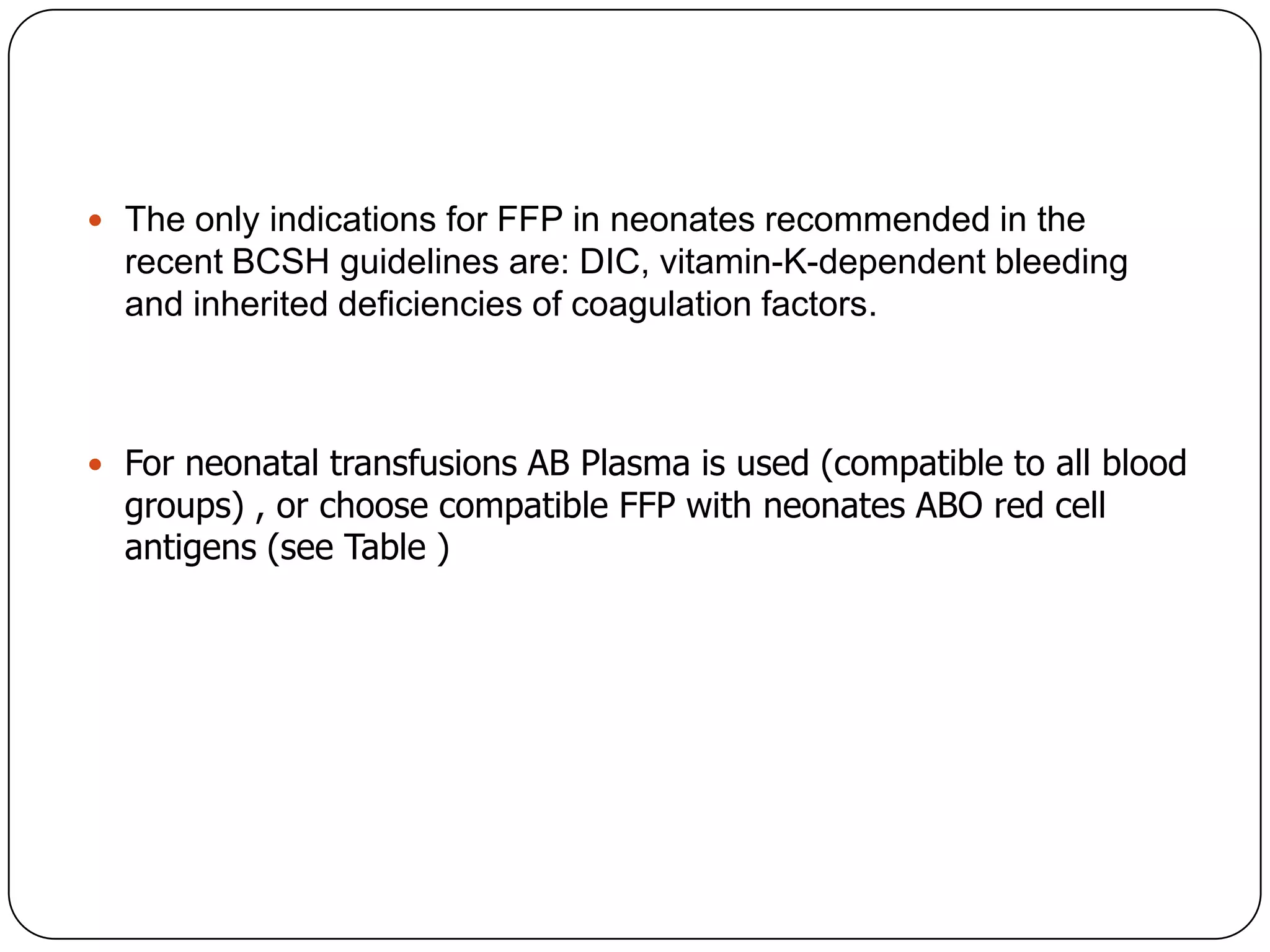
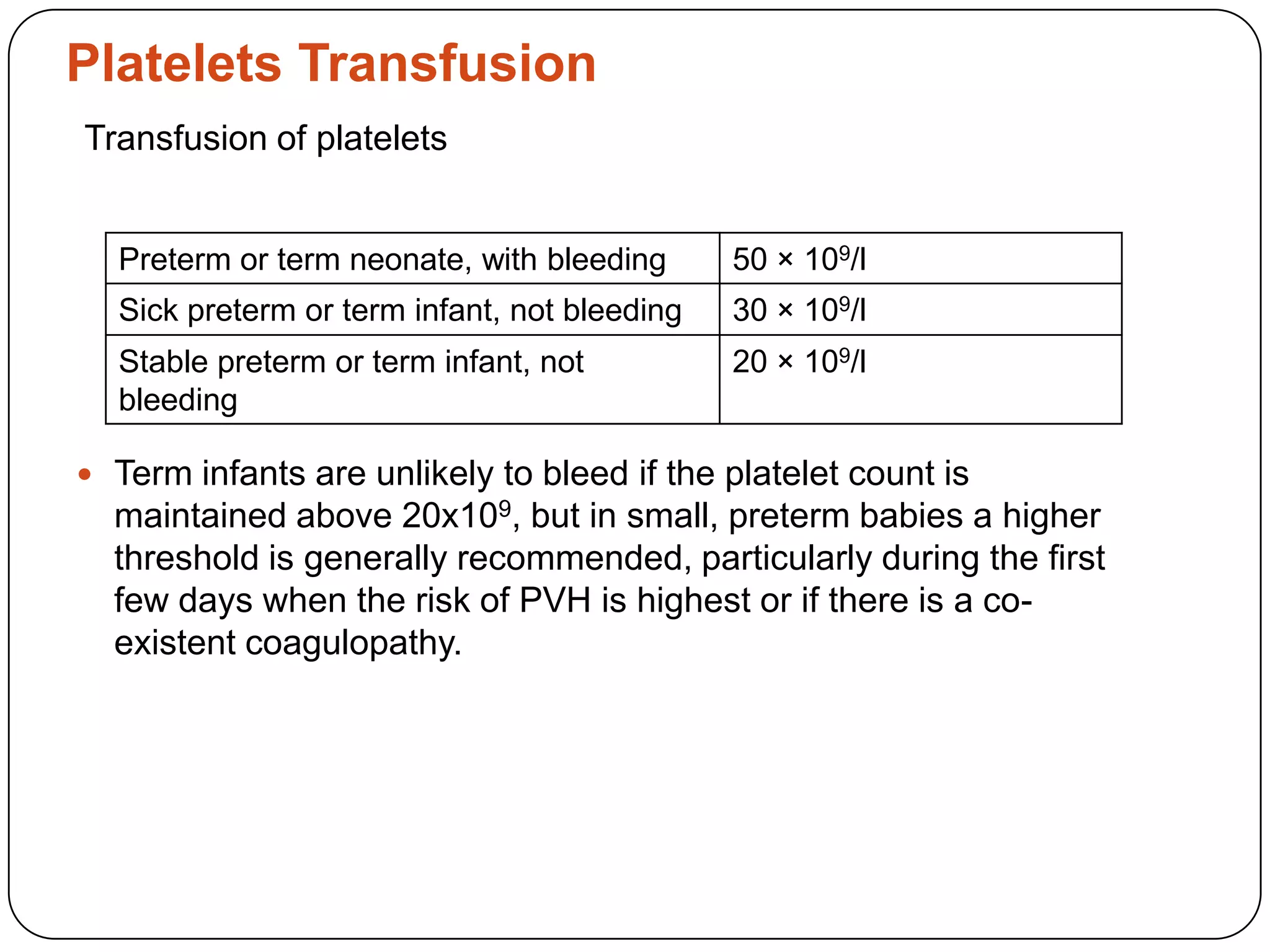
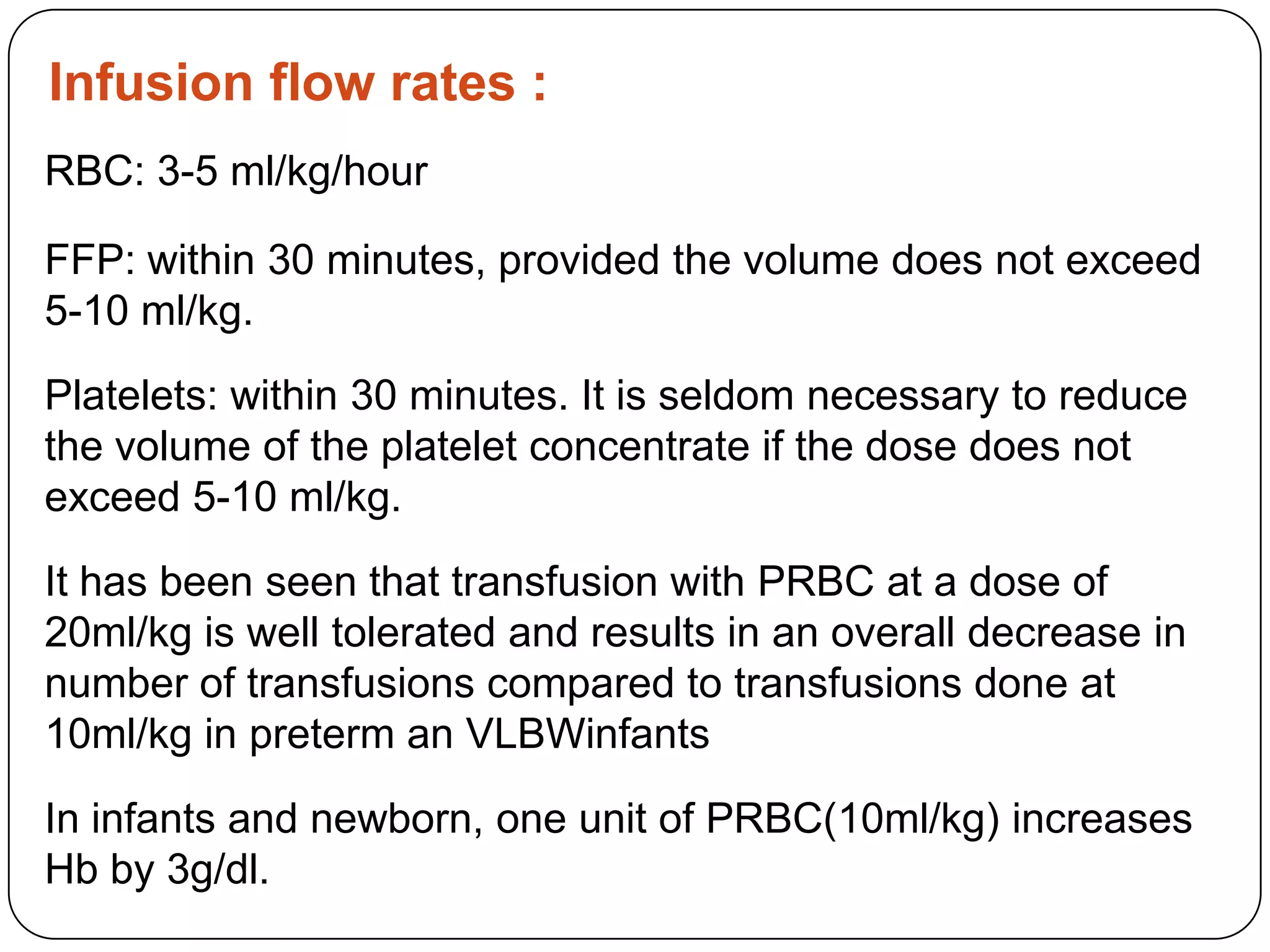
Blood transfusion in neonates carries risks and should only be done when benefits outweigh risks. Special considerations for neonates include their small size, immature immune systems, and unique hemoglobin and erythropoiesis characteristics. Clinical guidelines can help standardize best practices to improve outcomes. Strategies to reduce transfusions include delayed cord clamping and restrictive blood sampling. Proper blood component specification, testing, and product selection are crucial to ensure safety.