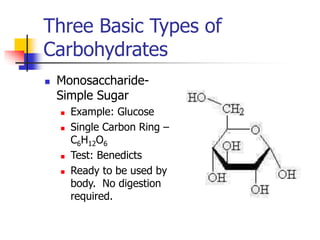
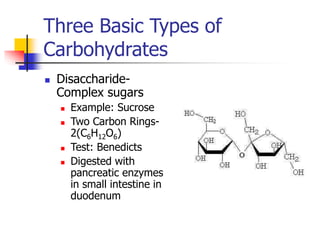
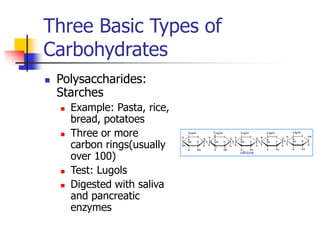
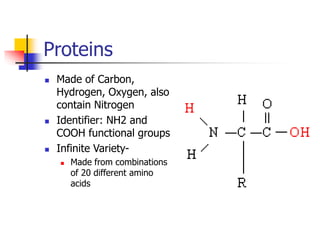
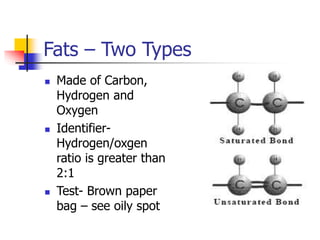
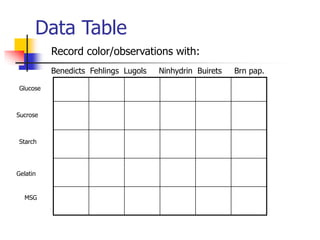
This document discusses the three main types of biomolecules - carbohydrates, proteins, and fats. Carbohydrates include monosaccharides like glucose, disaccharides like sucrose, and polysaccharides like starch. Proteins are made from combinations of 20 different amino acids. Fats can be saturated or unsaturated, and are made from glycerol and fatty acids. A variety of tests can identify these biomolecules, such as Benedict's solution for carbohydrates or ninhydrin for proteins.