This document provides an introduction to the development of a 3D in vitro tumor model. It discusses some of the key questions that guided the investigators in their research, such as how cells behave differently in 3D versus 2D environments, and how the extracellular matrix microenvironment can impact cell behavior and shape. The researchers chose to focus on metastatic uveal melanoma cells, as these tumors form unique patterns (vasculogenic mimicry patterns) in the liver. Their model utilized these cancer cell lines seeded within extracellular matrix proteins to mimic the human body's 3D structure and mechanical tensions. Their work built upon the concept of "tensegrity" and helped demonstrate how the extracellular environment can differentially stabilize DNA exposure and influence gene




![PLATELET CYTOKINESIS AND LACK OF THROMBOSIS IN
LAMININ- LINED VM PATTERNS………………………………………… 34
A. Materials and methods ………………………………………………. 38
1. Platelet acquisition and preparation ……………………… 38
2. Three dimensional matrix-containing cultures ………….. 38
3. Observation and data capture ……………………………. 38
B.Results…………………………………………………………….….. 38
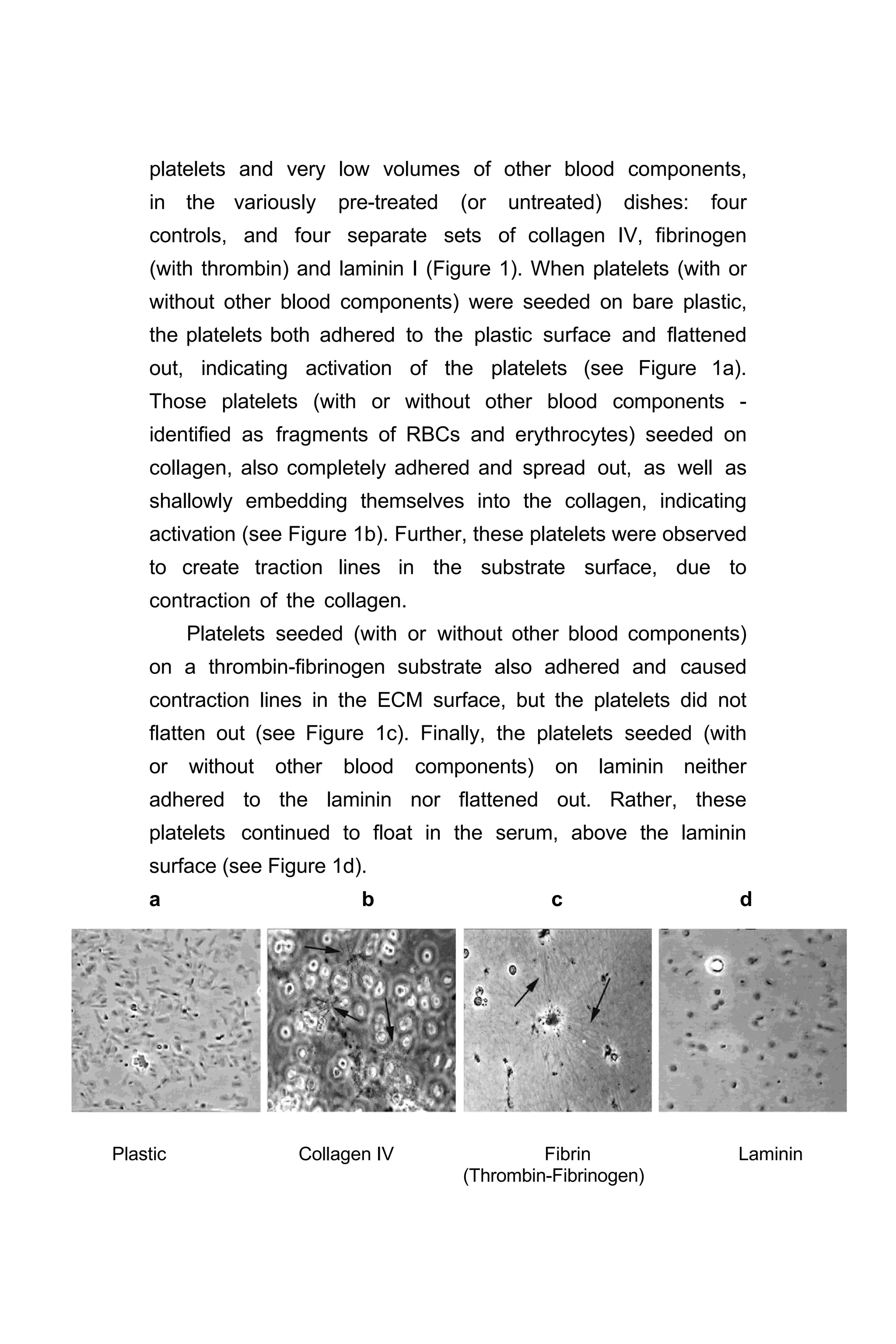
1. Platelet adhesion/activation and ECM substrates ….….. 38
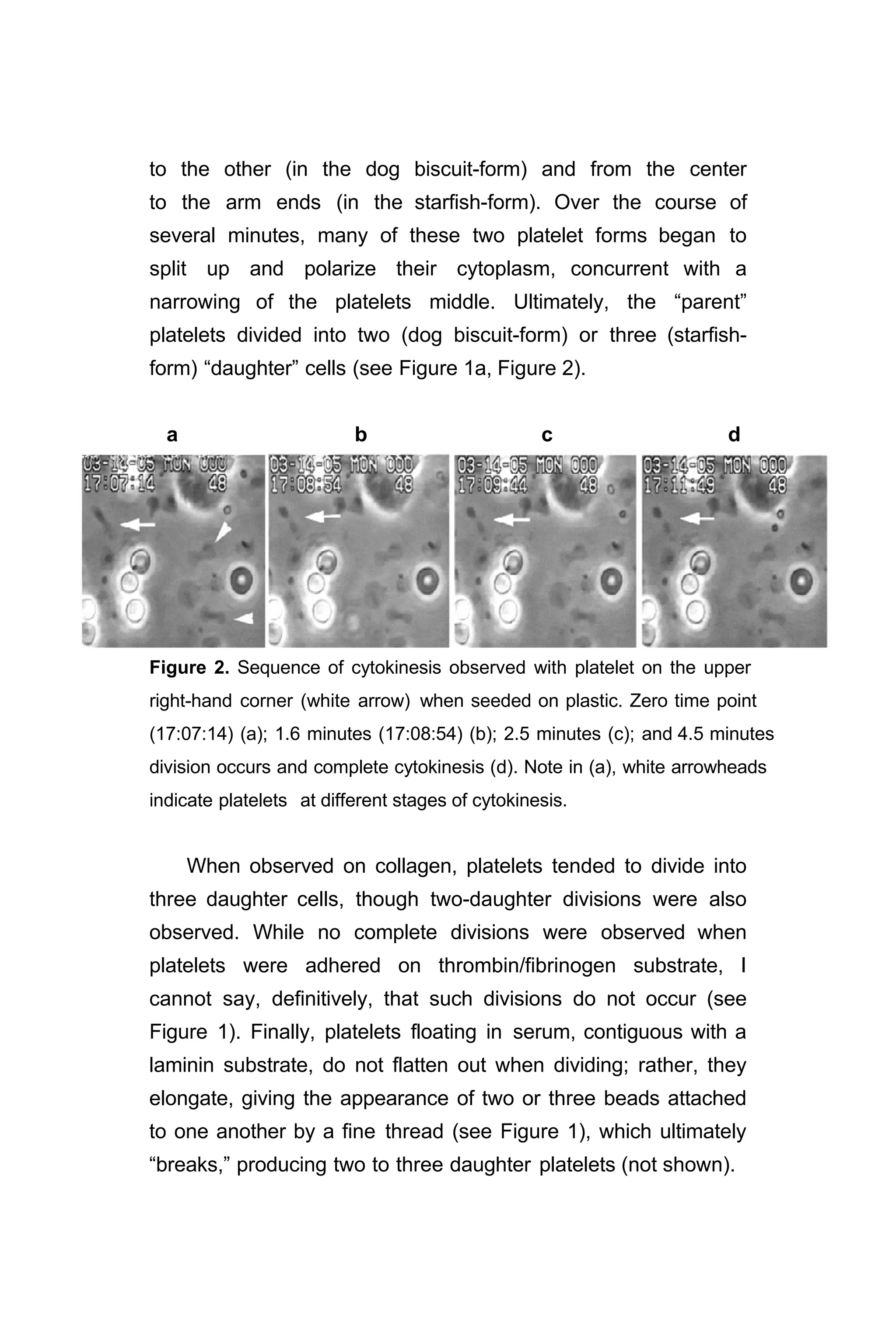
2. Platelet division …………………………………………….. 40
C.Discussion……………………………………………………………. 41
D. Acknowledgments …………………………………………………… 43
E. References …………………………………………………………… 44
IV.CONCLUSION………………………….……………………………………. 46
A. Highlights of observations, discoveries, outcomes
and implications………………………………………………………… 48
B. Potentialfutureinvestigation………………………………………….... 50
C. Cytoplast and Platelet Findings in the Context of the Bio-
engineeredTumorModel……………………………………………… 52
FIGURES
CHAPTER PAGE
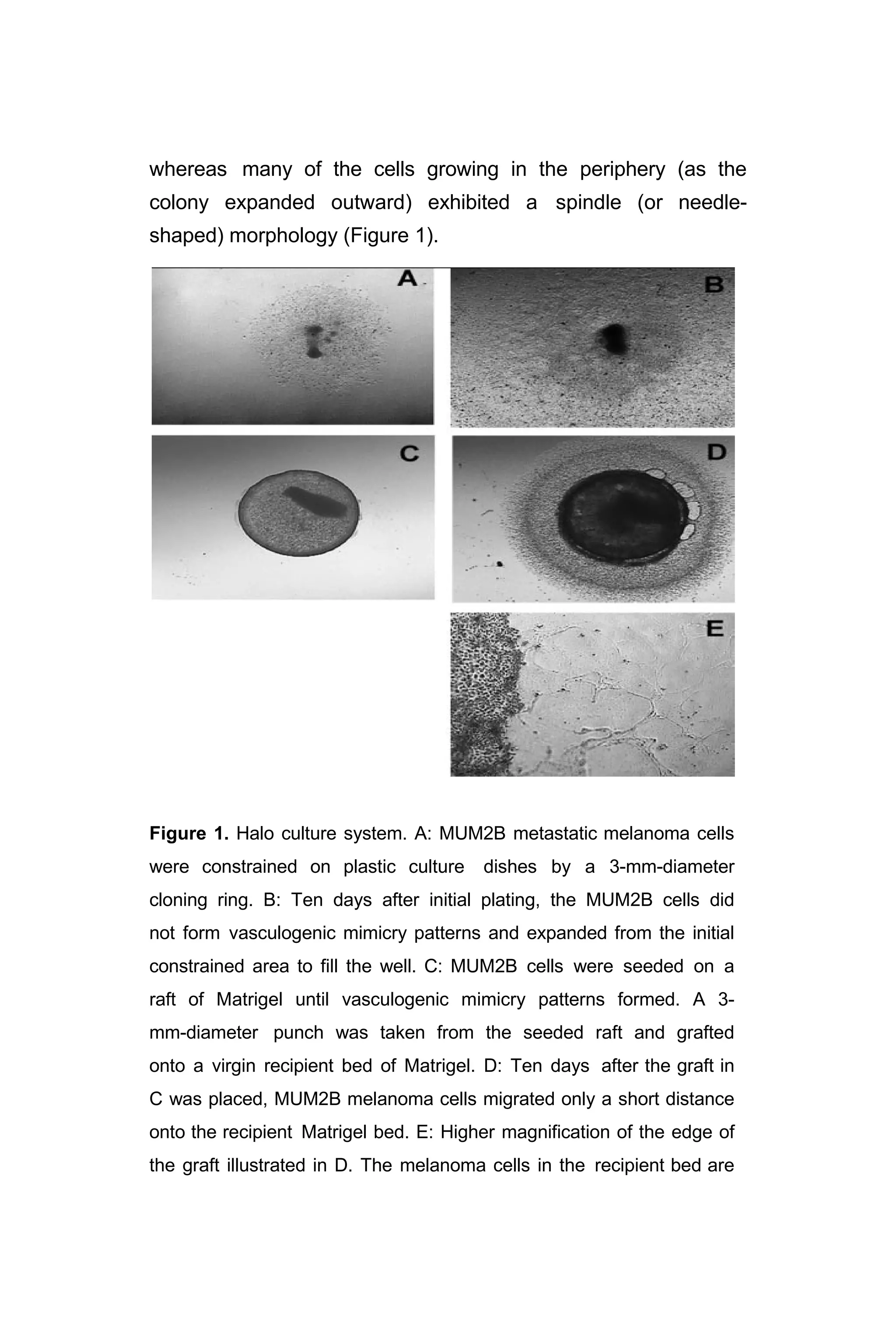
I. Figure 1: “Halo Culture System” ……………………….…………… 12
Figure 2: “Low-density cultures of MUM2B cells
in Matrigel followed throughout 10 days”………….. 14
Figure 3: “Differential killing of metastatic versus spindle A
and B [?] cells in metastatic melanoma 3-dimenional
vasculogenic mimicry cultures ………………….. 16
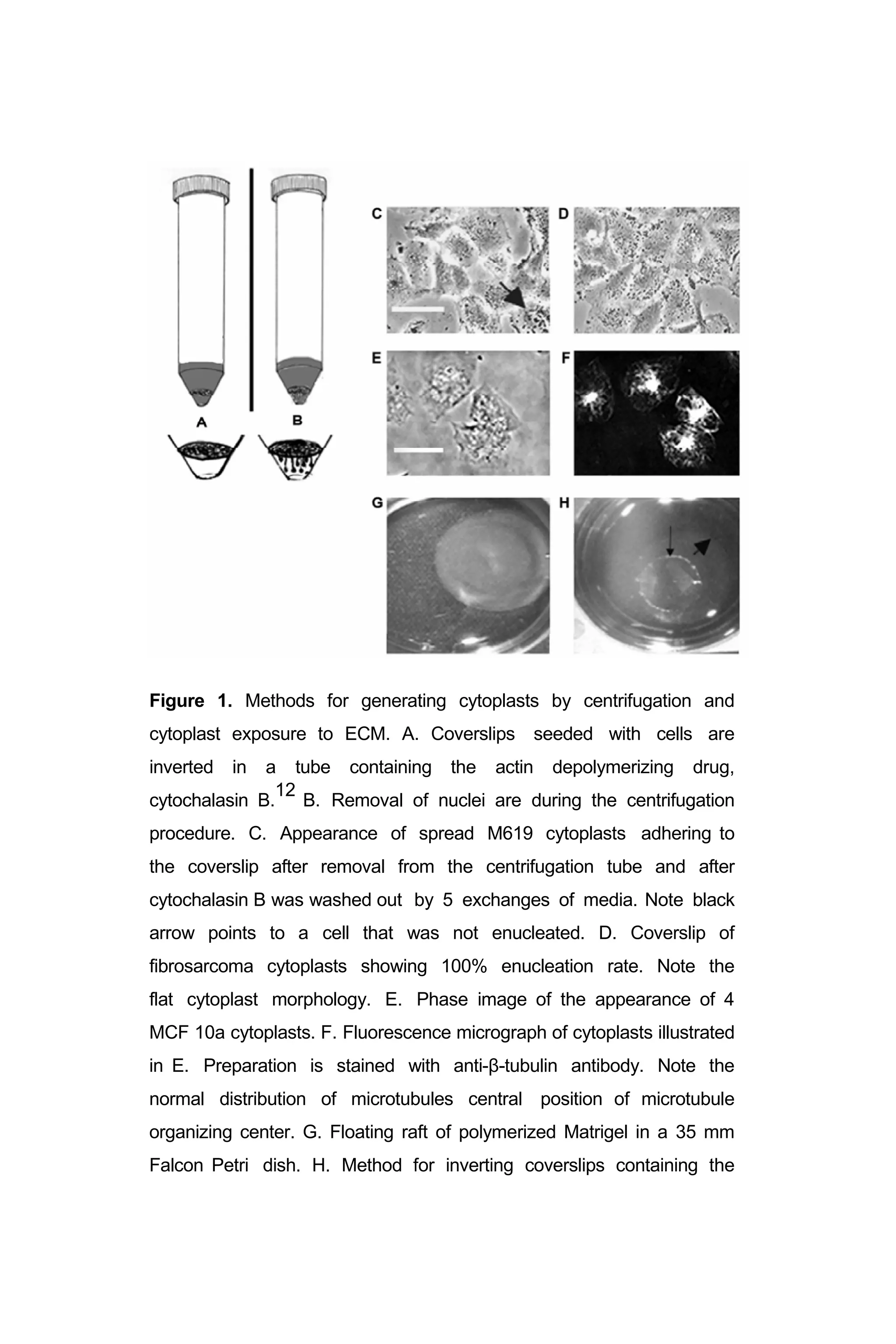
II. Figure 1: “Methods for generating cytoplasts by centrifugation
And cytoplast exposure to ECM”………………………………… 22](https://image.slidesharecdn.com/06e5761e-2d08-47e1-9280-e15b270603a6-161116205012/75/BioengineeredTumorModel_DrJonasMoses_r2015-5-2048.jpg)










![when drugging these cultures with certain analogs: the cells
closest to the perimeter of a VM channel (which appear to
have reverted from a metastatic morphology to a spindle A
type cell) are unaffected by the analog, while the cells furthest
away from the loop edge (and also furthest from the laminin or
Matrigel substrate), exhibiting a typically epithelioid morphology
(signal of highly invasive tumor cells) round up and die (Figure
3). Cell death was established by staining: based on
exclusion or incorporation of Trypan blue.9
A B
C D
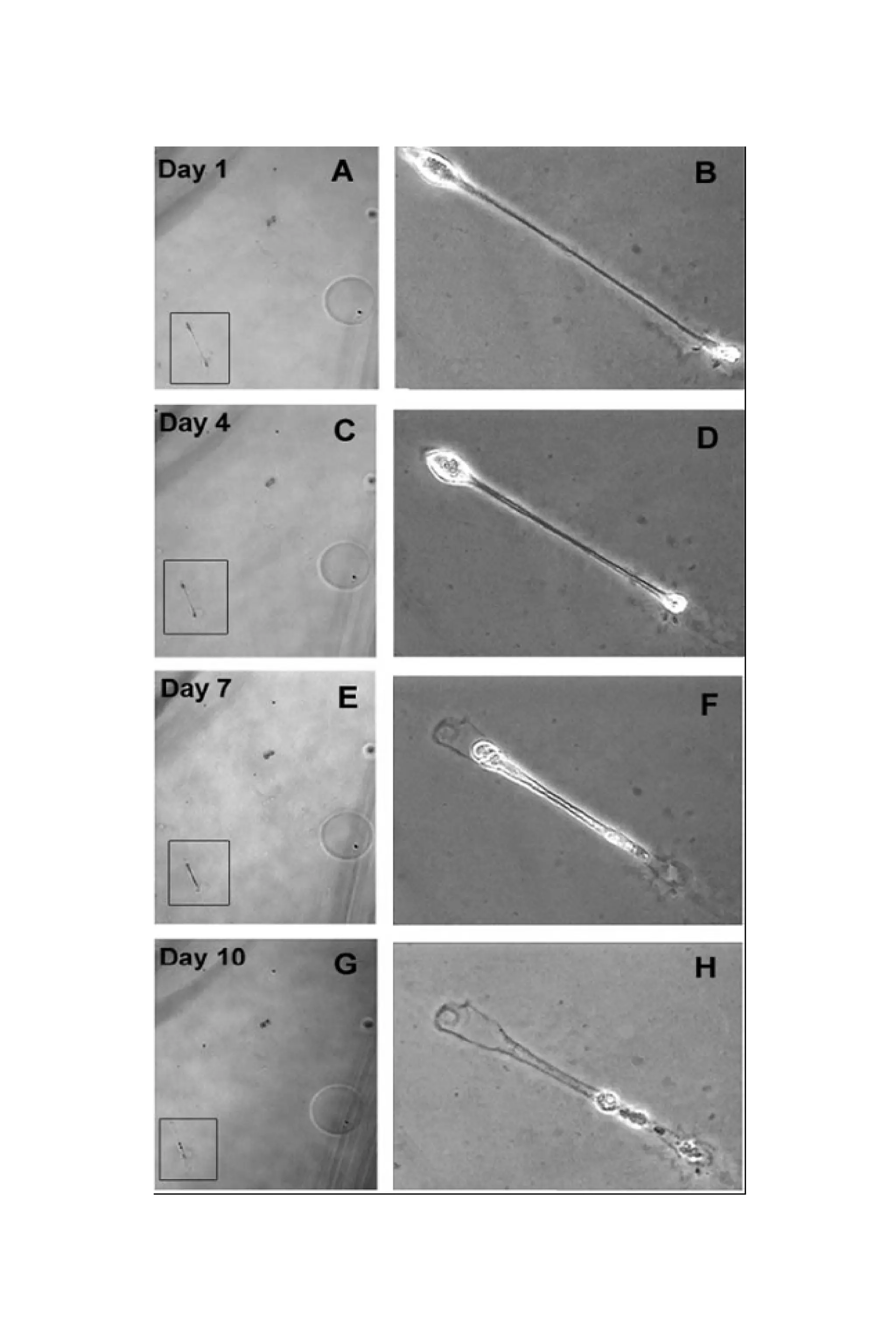
Figure 3. A-D: Differential killing of metastatic versus spindle A and B
[?] cells in metastatic melanoma 3- dimensional vasculogenic mimicry
cultures. MUM2B spindle A and epithelioid cells form looping patterns
in mature cultures, and are then either untreated (A,B), or treated
(C,D) with CGC-11144. After 24 hours of incubation, Trypan dye is](https://image.slidesharecdn.com/06e5761e-2d08-47e1-9280-e15b270603a6-161116205012/75/BioengineeredTumorModel_DrJonasMoses_r2015-19-2048.jpg)







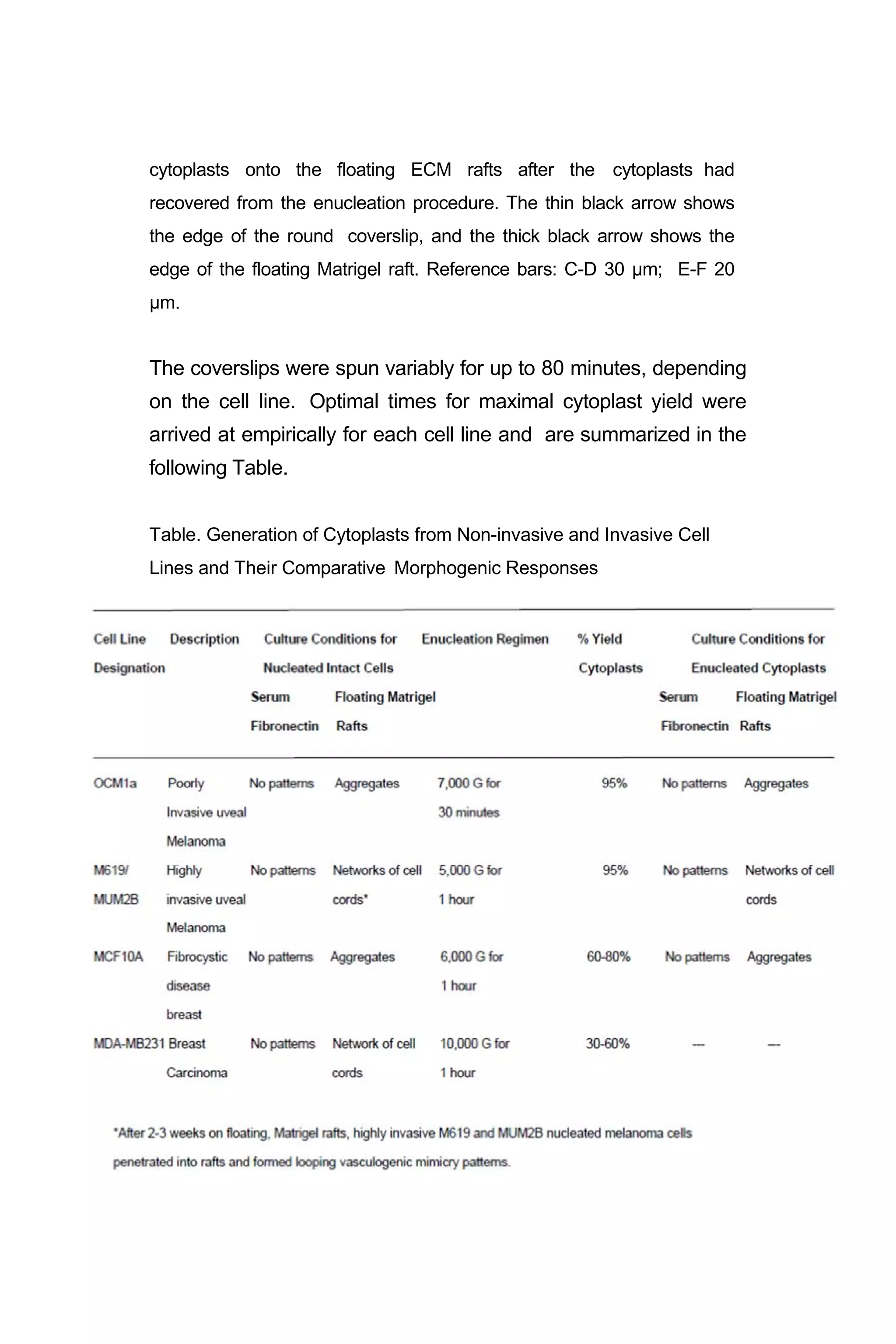



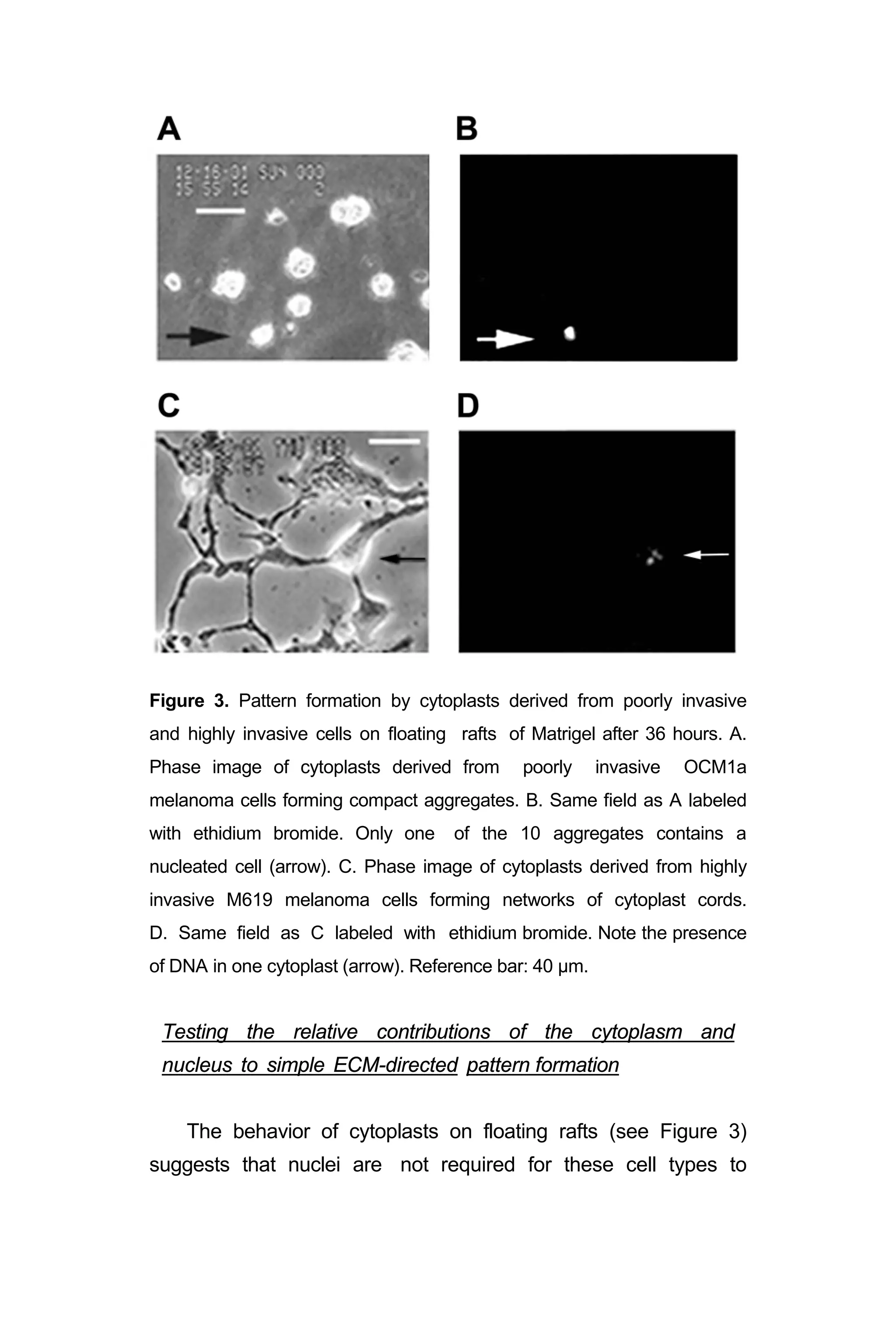
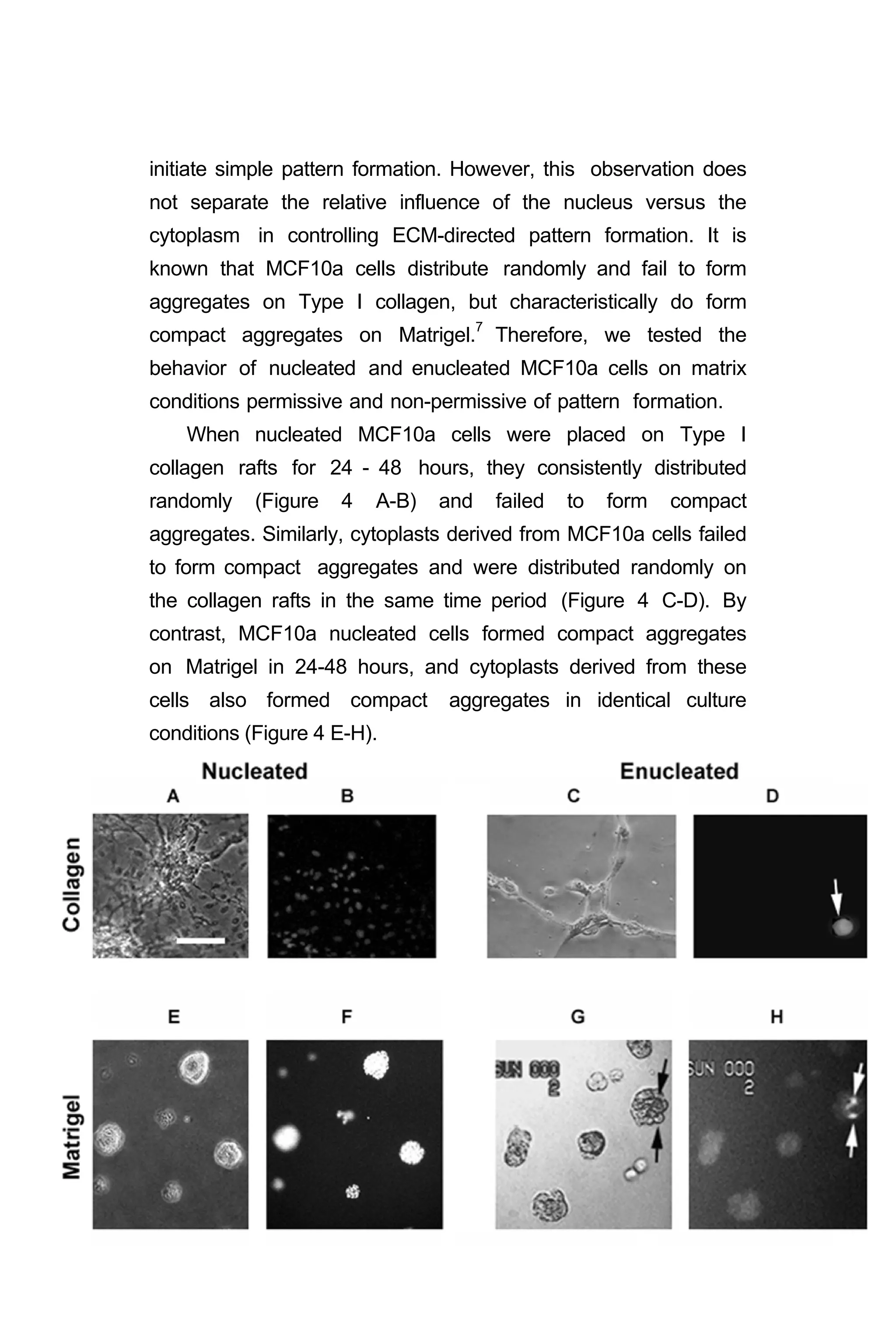
![Figure 4. Testing the relative contributions of ECM and nuclei to simple
pattern formation. A. Phase image of poorly invasive MCF 10a
nucleated cells from fibrocystic disease of the breast dispersed on
collagen Type I. B. Fluorescence micrograph of same field in A labeled
with ethidium bromide. C. Phase image of poorly invasive MCF 10a
enucleated cytoplasts dispersed on collagen Type I. D. Fluorescence
micrograph of same field in C labeled with ethidium bromide. Only one
of the cells contains DNA. E. Phase image of poorly invasive MCF 10a
nucleated cells forming compact aggregates on Matrigel. F. Fluorescence
micrograph of same field in E labeled with ethidium bromide. [Ethidium
bromide, which fluoresces when exposed to UV light, binds to nuclear
DNA; and, both demonstrates the presence of DNA in the nucleus and
causes the bound DNA to become brittle.] G. Phase image of
poorly invasive MCF 10a enucleated cytoplasts forming compact
aggregates on Matrigel. H. Fluorescence micrograph of same field in G
labeled with ethidium bromide. One of the 8 aggregates contains two
cells with DNA. Reference bar a,b = 60 µm; c,d = 30 µm; e-h = 60 µm.
Discussion
This study was designed to identify the relative contributions
of the cytoplasm and the nucleus in the generation of simple
ECM-induced morphogenetic patterns (cord formation and
spherical aggregates) by poorly invasive cells and highly
invasive cells. Members of our investigative team,3
and others,7
had shown previously that nucleated cells of varying invasive
potential - normal fibroblasts and endothelial cells, poorly
invasive cells breast epithelial cells, poorly invasive melanoma
cells, and highly invasive fibrosarcoma, melanoma, and breast
carcinoma - do not form spheroidal nests, cords, or networks
when plated on fibronectin adsorbed to glass coverslips.](https://image.slidesharecdn.com/06e5761e-2d08-47e1-9280-e15b270603a6-161116205012/75/BioengineeredTumorModel_DrJonasMoses_r2015-35-2048.jpg)