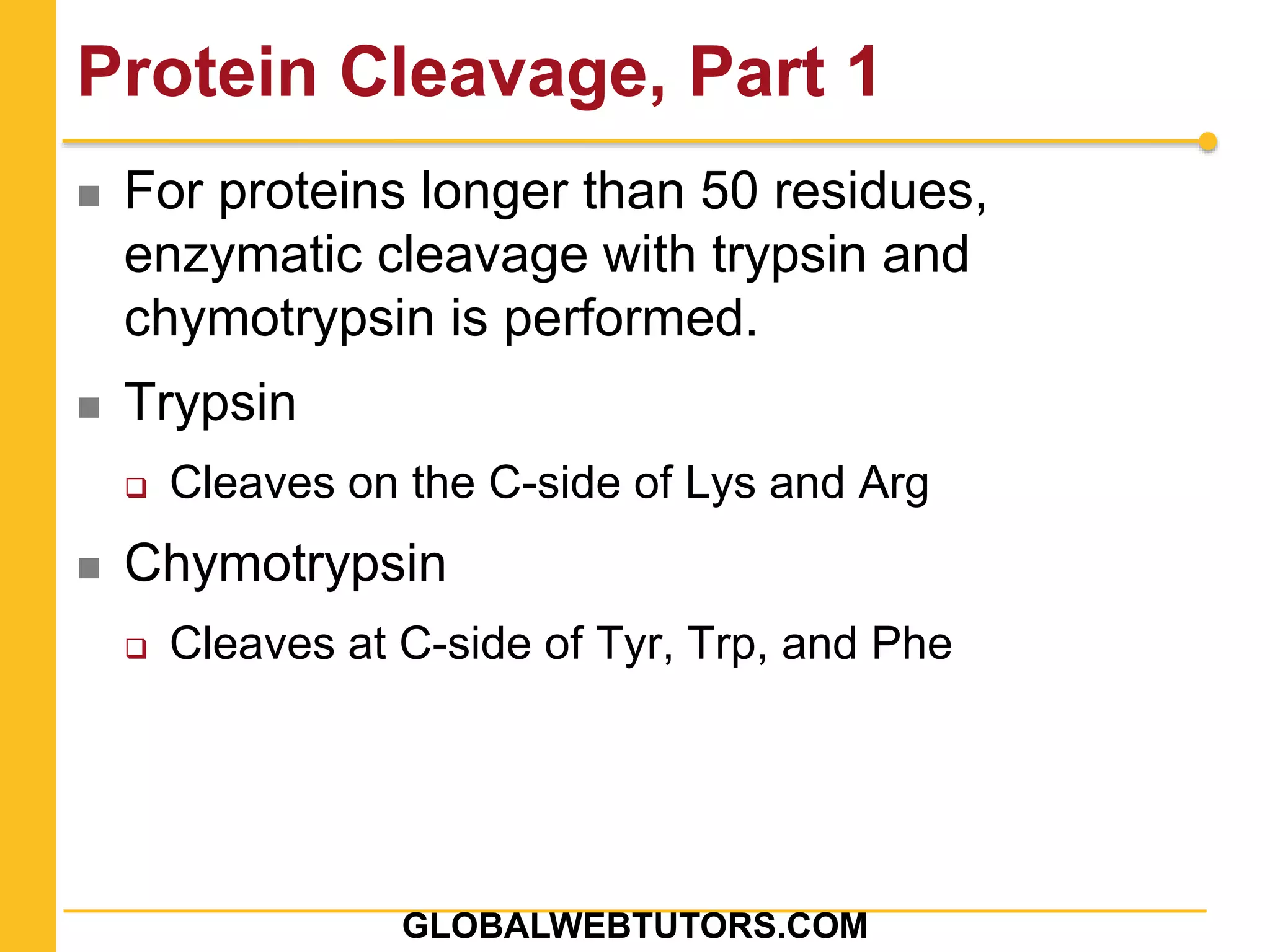
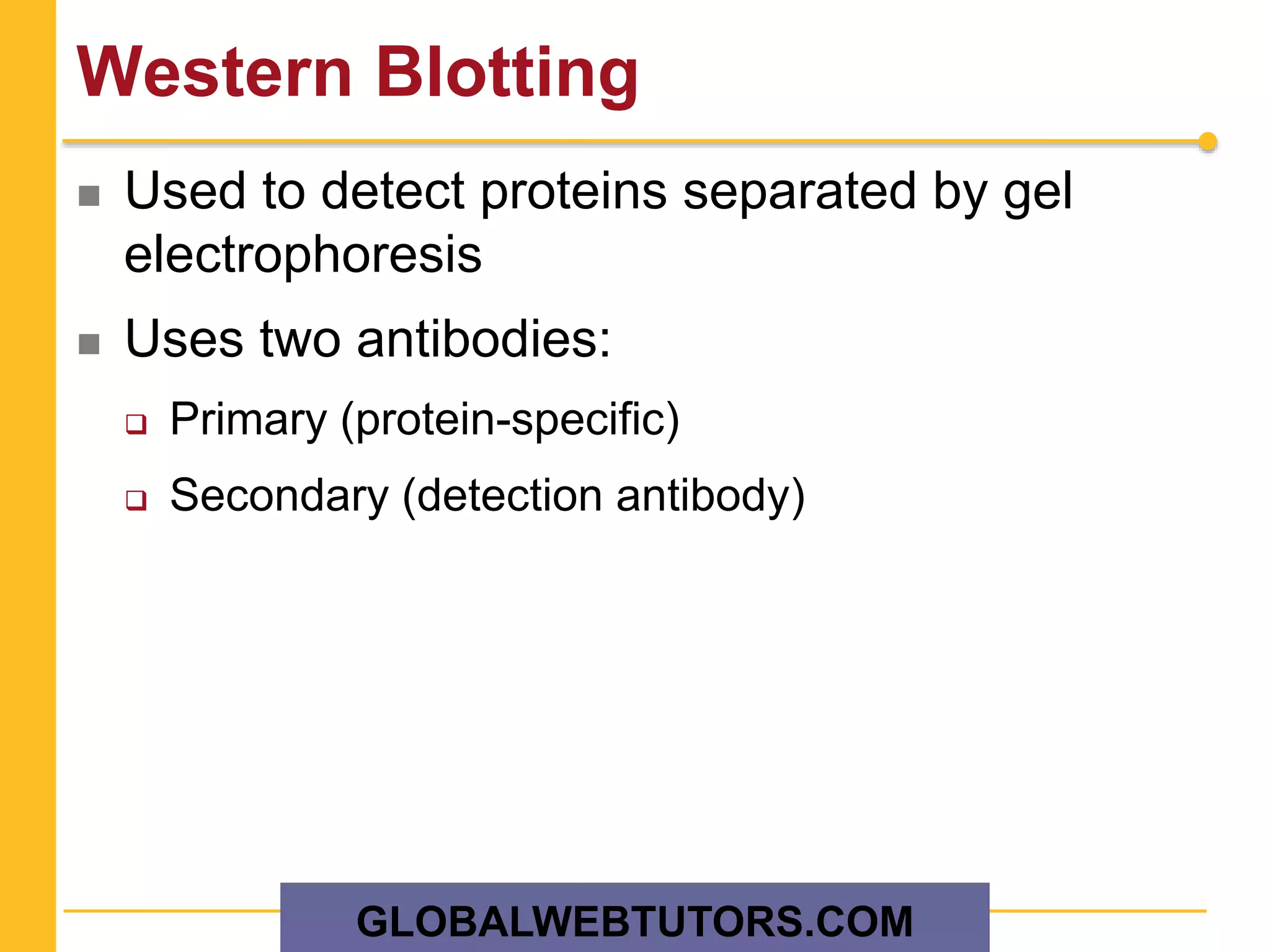
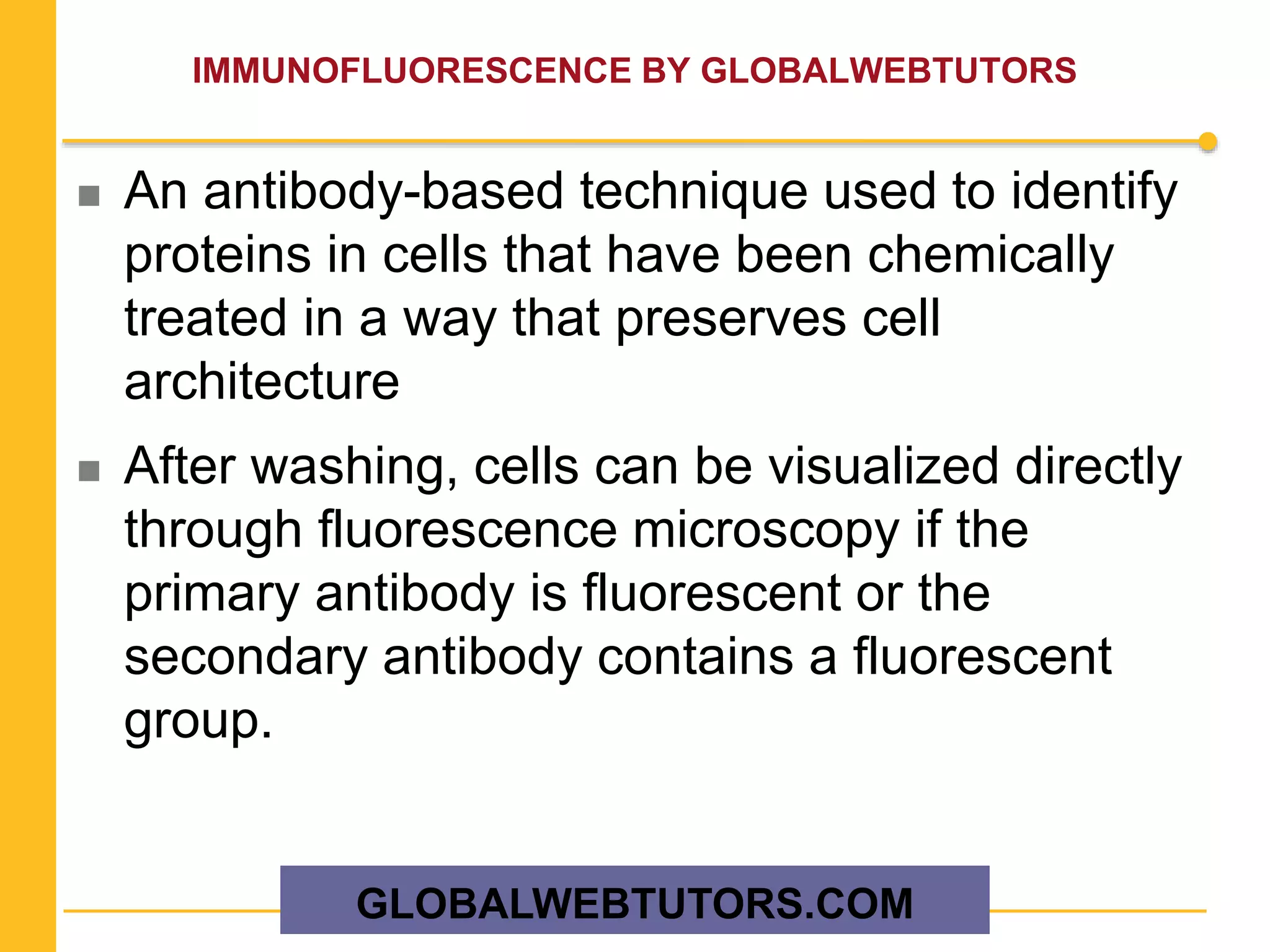
The document provides an overview of various methods in protein biochemistry, including protein purification techniques, cell fractionation, chromatography methods, and protein characterization through assays like gel electrophoresis and western blotting. It discusses specific approaches for sequencing proteins and the use of antibodies in detecting and isolating proteins. The document highlights the importance of exploiting biochemical properties for effective protein analysis and purification.