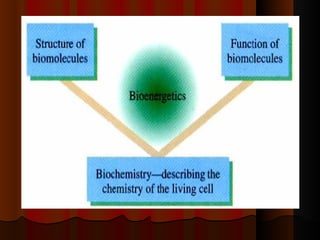
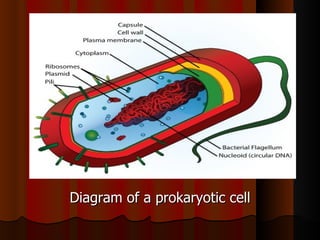
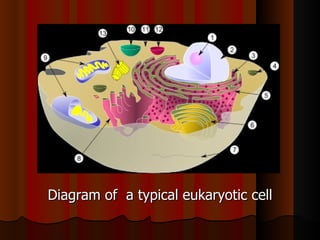
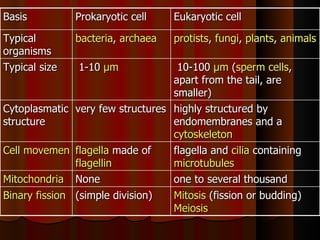
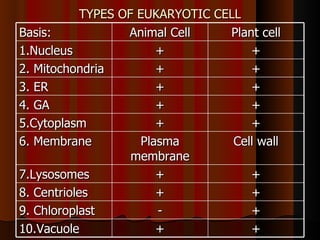
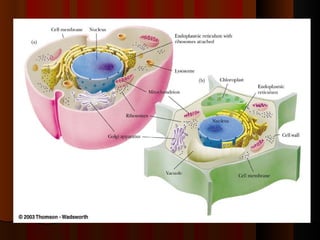
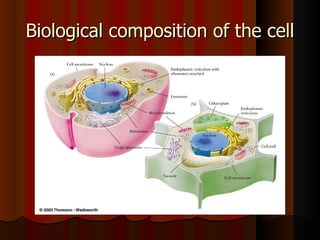
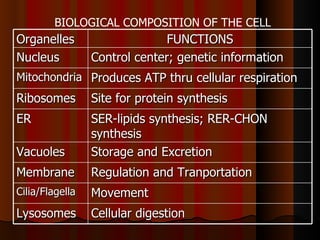
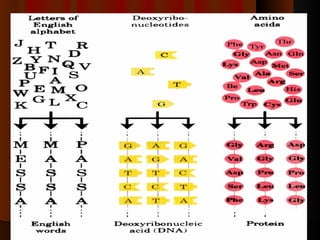
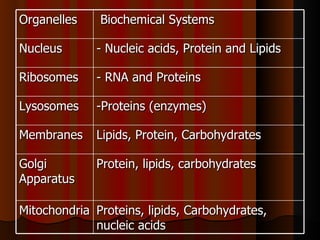
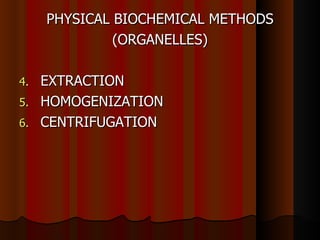
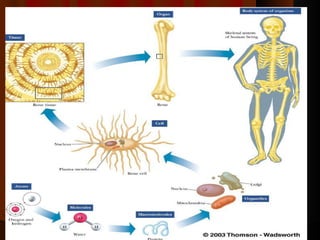
Biochemistry is the study of life at the molecular level, examining the structure, organization and functions of living organisms. It seeks to understand life through studying biological molecules like proteins, carbohydrates and nucleic acids. The cell is the basic unit of life, and biochemistry examines both eukaryotic and prokaryotic cells. Cells contain complex biological molecules that carry out essential functions through various organelles and biochemical pathways.