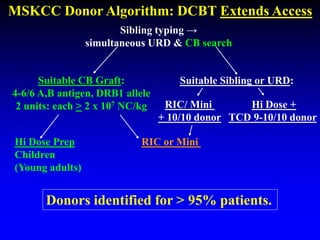
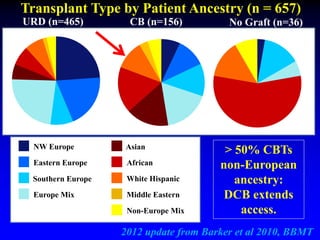
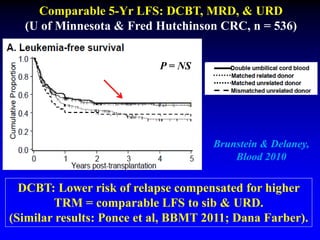
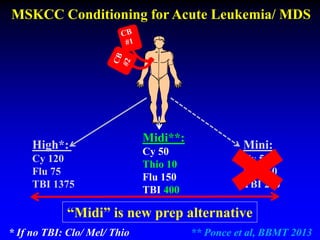
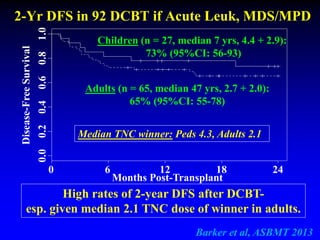
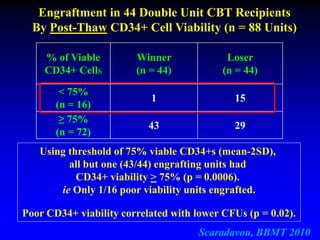
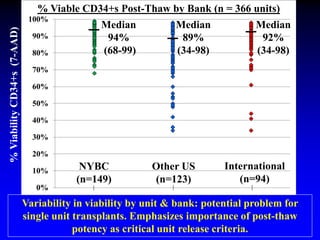
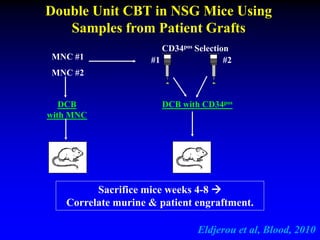
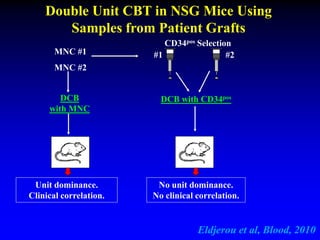
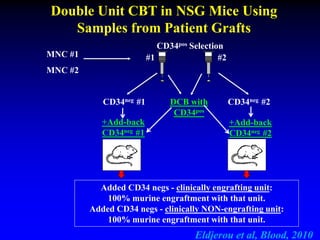
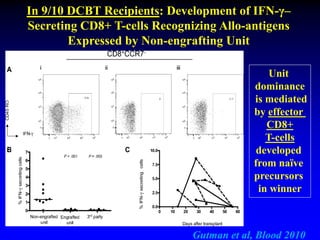
This document summarizes research presented by Dr. Juliet Barker on double unit cord blood transplantation (DCBT) for acute leukemia. The key points are:
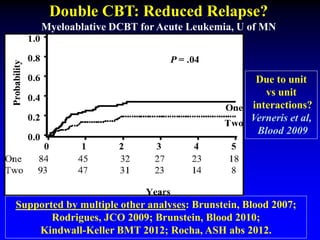
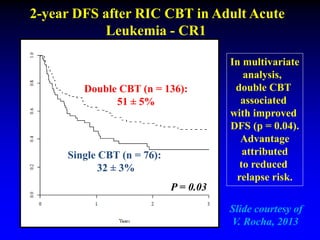
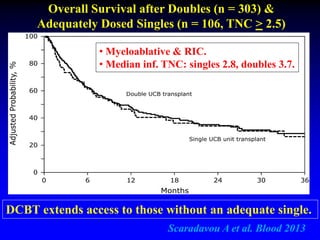
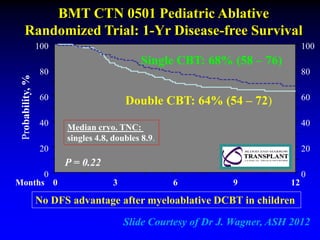
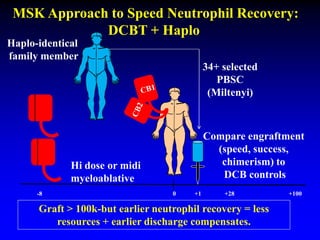
1. DCBT aims to augment graft cell dose to improve engraftment, reduce transplant-related mortality, and improve survival rates compared to single unit CBT.
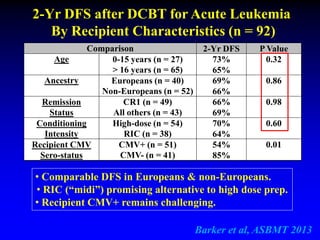
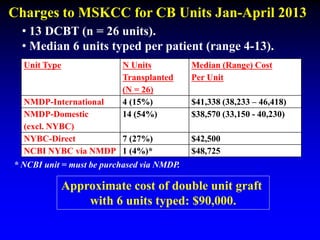
2. Studies at MSKCC and other centers show comparable or improved survival rates for DCBT compared to matched related or unrelated donor transplants.
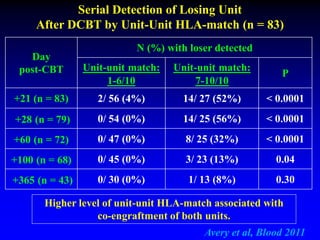
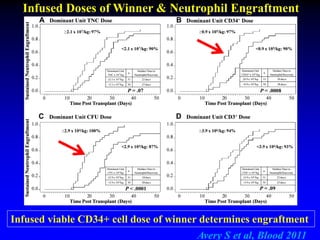
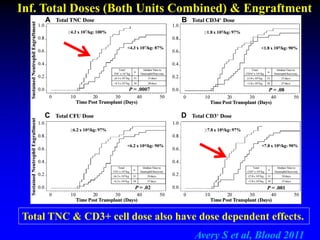
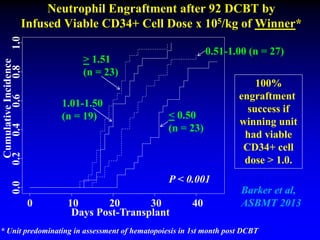
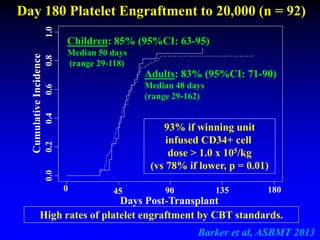
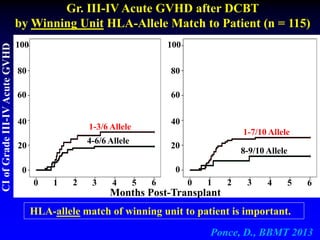
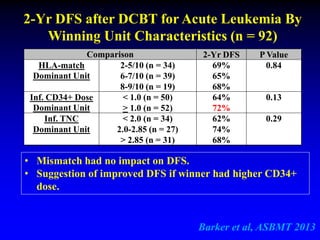
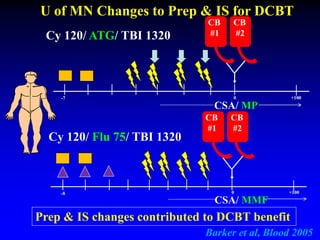
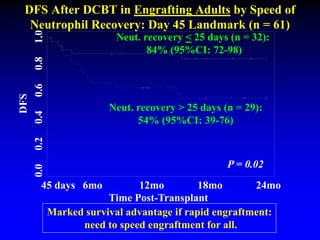
3. Factors that influence engraftment success include the infused dose of CD34+ cells from the winning cord blood unit and the HLA match between the units.
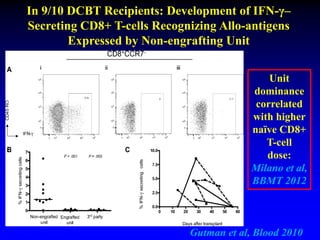
4. Research suggests the winning unit is determined by immune