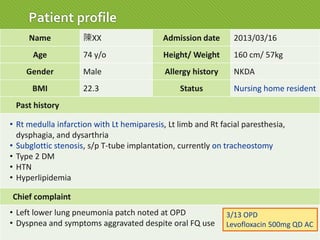
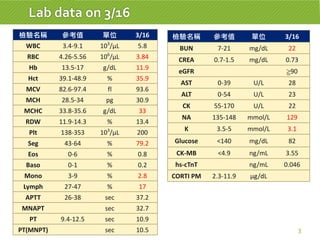
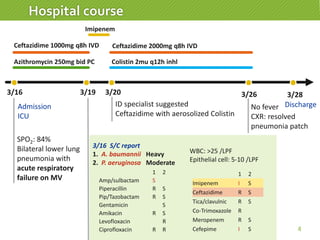
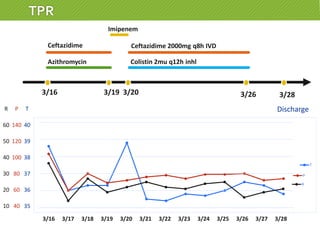
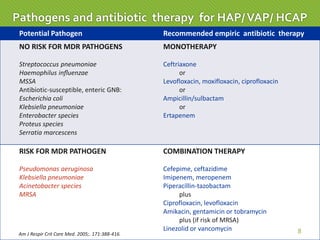
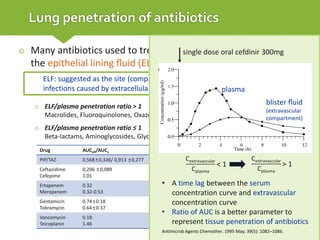
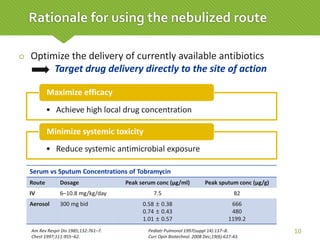
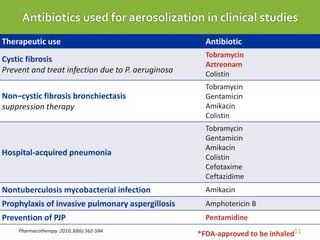
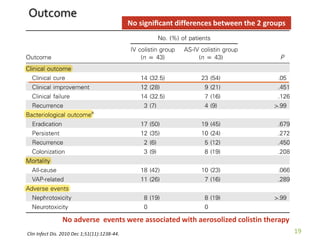
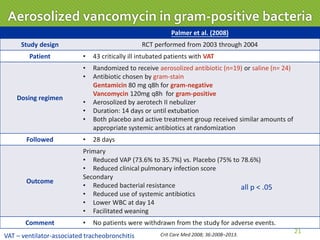
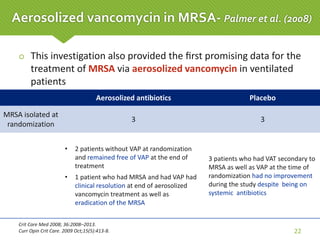
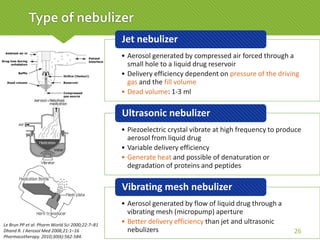
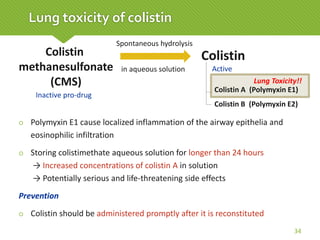
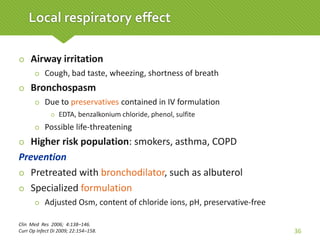
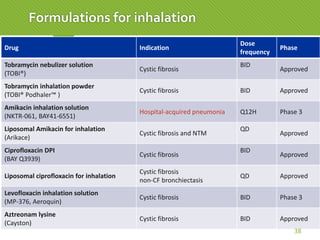
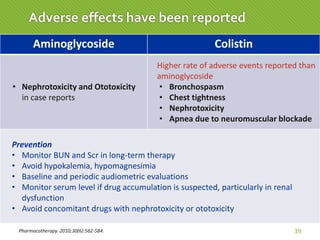
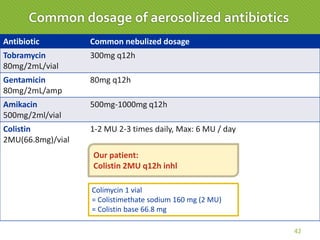
This case report describes a 74-year-old male nursing home resident admitted for left lower lung pneumonia. His medical history includes right medulla infarction with left hemiparesis, subglottic stenosis post tracheostomy, diabetes, hypertension, and hyperlipidemia. Laboratory tests and imaging show bilateral lower lung pneumonia with acute respiratory failure requiring mechanical ventilation. Sputum cultures grow Acinetobacter baumannii and Pseudomonas aeruginosa resistant to many antibiotics. The patient is treated with a combination of intravenous antibiotics including ceftazidime, azithromycin, imipenem, and nebulized colistin, with resolution of symptoms and pneumonia.