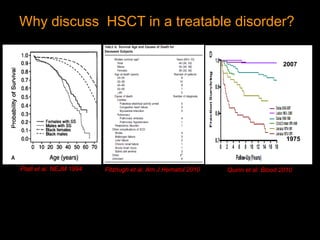
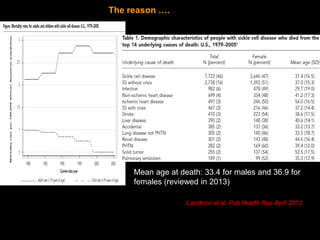
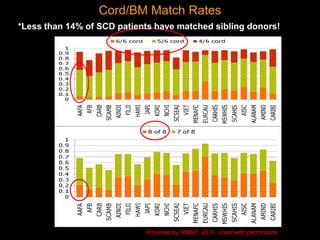
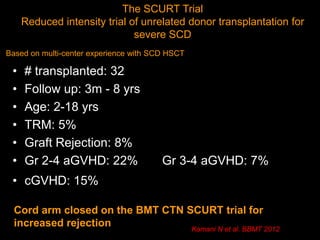
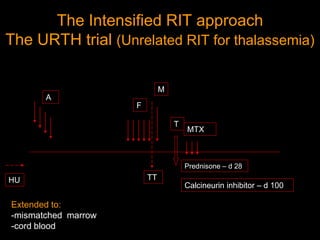
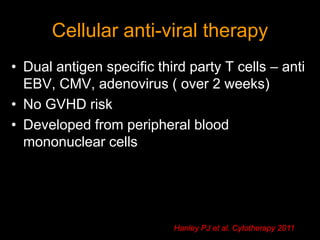
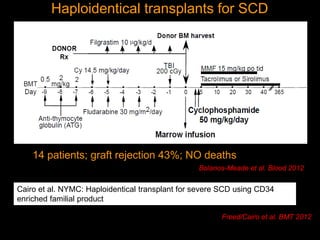
1) Hematopoietic stem cell transplantation (HSCT) is being explored as a potentially curative treatment for sickle cell disease (SCD) due to the severe complications associated with SCD including stroke, acute chest syndrome, chronic pain, and early mortality.
2) While HSCT can cure SCD by replacing the defective hematopoietic stem cells, current transplant approaches still carry high risks of transplant-related mortality, graft-versus-host disease, and long-term effects on fertility and cognition.
3) Recent efforts aim to reduce the intensity of pre-transplant conditioning to lower toxicity while maintaining graft-versus-host disease prevention. Additional areas of research focus on optim