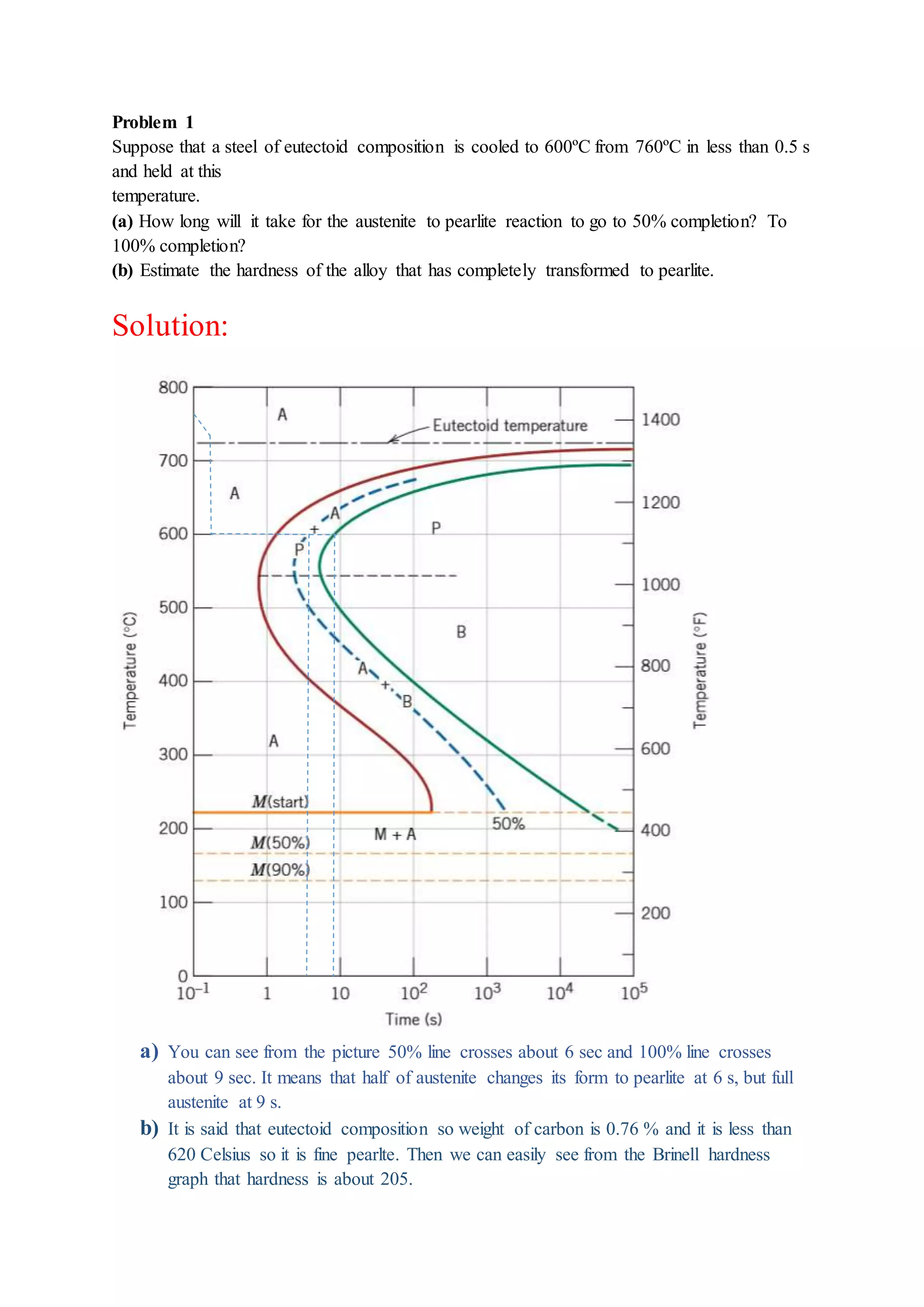
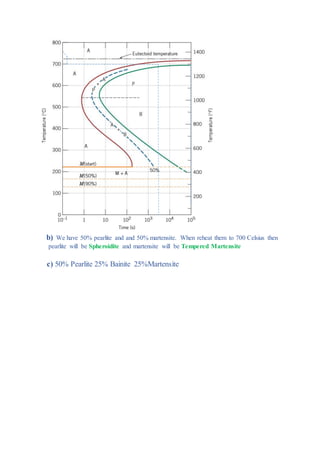
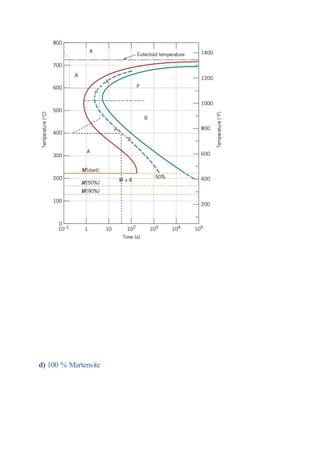
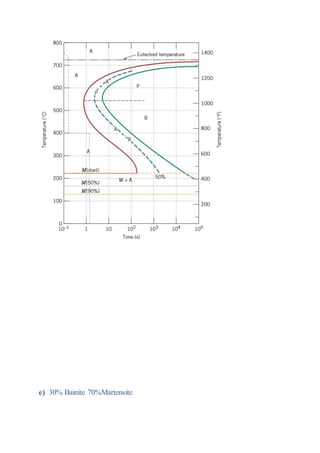
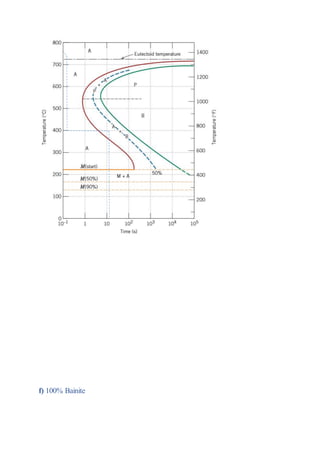
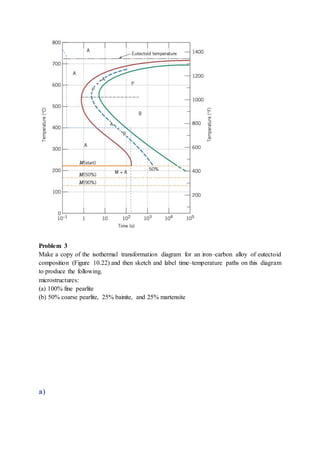
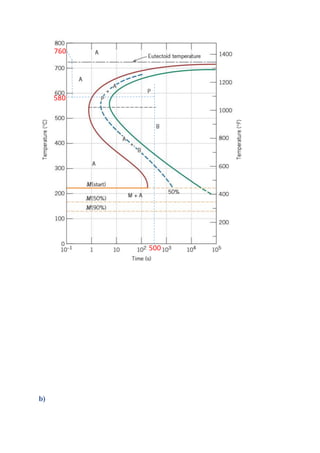
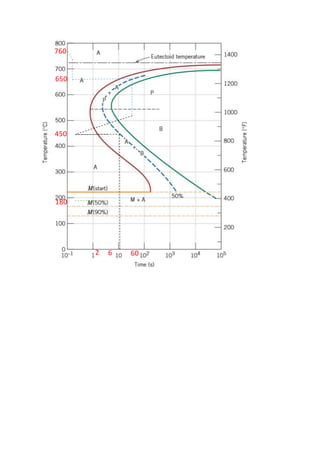
The document provides solutions to problems involving interpreting an isothermal transformation diagram for an iron-carbon alloy of eutectoid composition. In problem 1, it is determined that for 50% completion of the austenite to pearlite reaction it will take 6 seconds, and for 100% completion it will take 9 seconds. The hardness of fully transformed pearlite is estimated to be around 205 Brinell. Problem 2 specifies the microconstituents and percentages that would result from various time-temperature treatments. Problem 3 involves sketching time-temperature paths on the diagram that would produce 100% fine pearlite and a microstructure with 50% coarse pearlite, 25% bainite, and 25% martensite