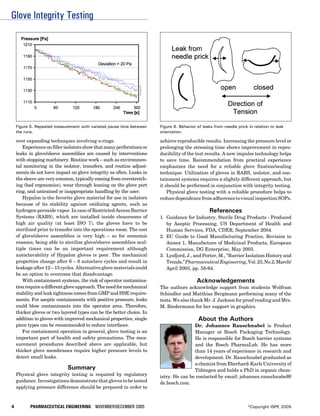
This document discusses procedures for testing the integrity of gloves used in barrier systems like isolators and RABS. It outlines shortcomings in detecting small leaks in gloves and presents a new impulse technique to improve reproducibility in pressure decay testing. Standard tests can detect leaks down to around 40 microns but not smaller leaks around 1 micron, which still pose contamination risks. A new impulse method is proposed to better evaluate the integrity of entire glove assemblies at a higher sensitivity.