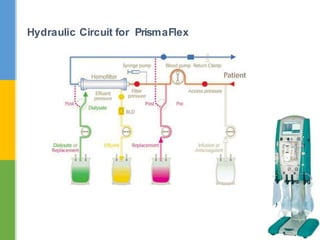
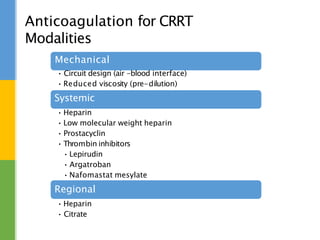
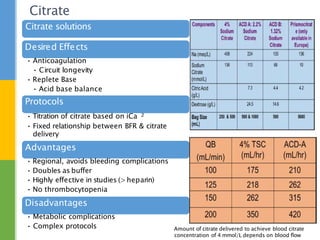
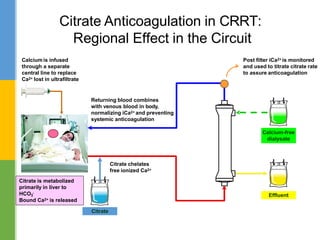
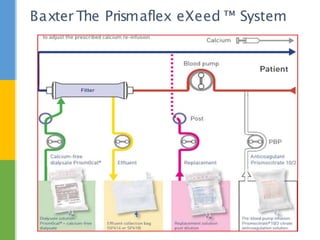
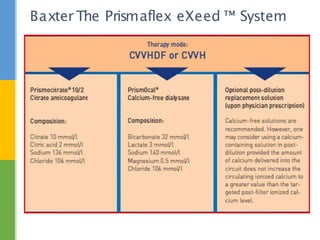
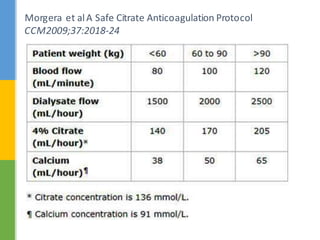
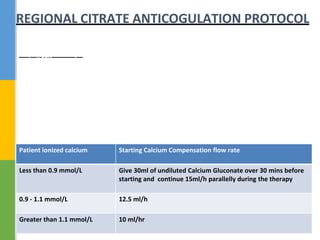
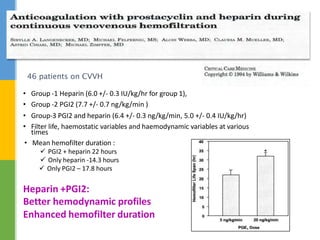
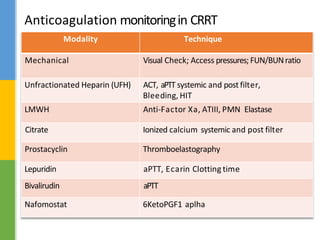
This document discusses anticoagulation for continuous renal replacement therapy (CRRT). It begins by outlining factors that can lead to clotting of CRRT filters and circuits. The main anticoagulation modalities discussed are heparin, low molecular weight heparin, citrate, and no anticoagulation. For each option, the mechanisms of action, advantages, disadvantages, dosing protocols, and typical filter life spans are summarized. Regional citrate anticoagulation is highlighted as it avoids systemic anticoagulation effects while effectively preventing clotting. Details are provided on citrate metabolism and calcium replacement to maintain safe ionized calcium levels.