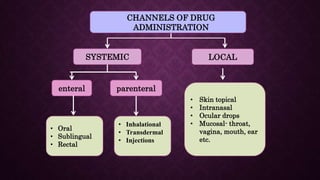
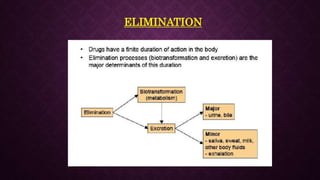
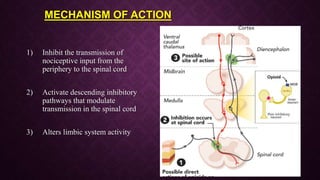
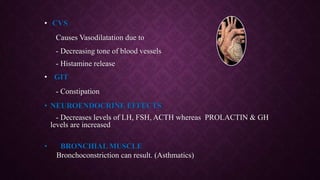
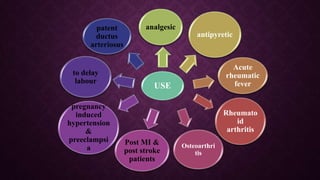
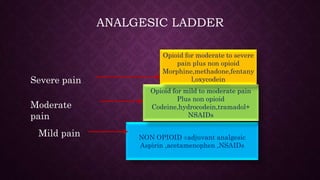
The document provides an extensive overview of pharmacology principles and analgesics used in dentistry, detailing routes of drug administration, pharmacokinetics, and pharmacodynamics. It covers analgesic types, including opioids and NSAIDs, their mechanisms of action, dosages for pediatric patients, and considerations for different patient populations. It concludes with discussions on managing pain in children and specific drug characteristics, usage guidelines, and potential side effects.