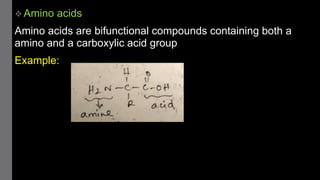
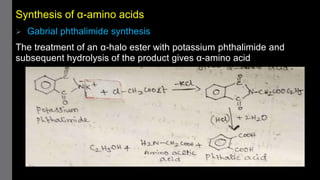
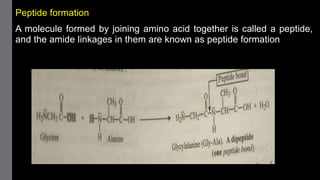
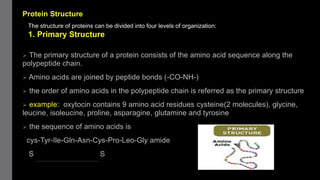
1) Amino acids are organic compounds that contain both amino and carboxyl groups. They can be classified as neutral, acidic, or basic depending on their side chains. Proteins are polymers of amino acids joined by peptide bonds.
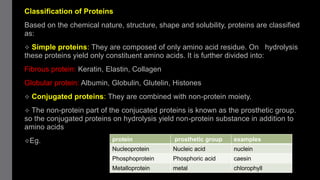
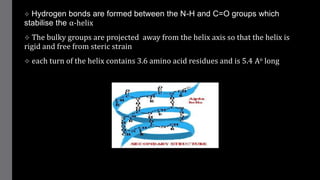
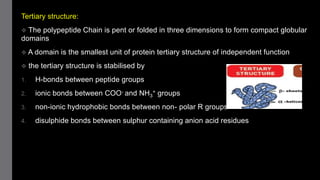
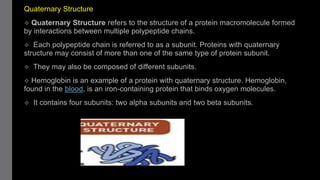
2) Proteins have primary, secondary, tertiary, and sometimes quaternary structures that determine their shape and function. They can be simple proteins composed only of amino acids or conjugated proteins bound to non-protein groups.
3) Common protein tests include the Biuret test which detects peptide bonds and the Ninhydrin test which detects amino acids.