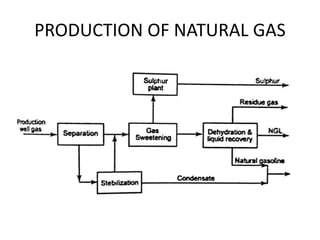
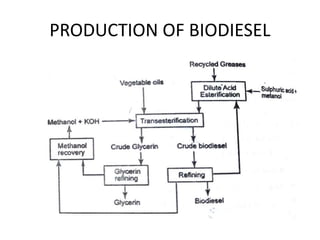
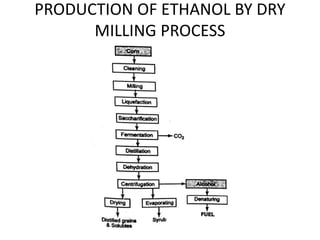
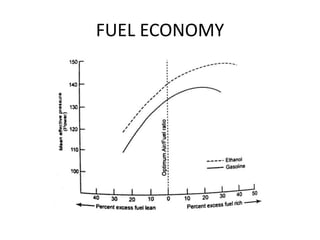
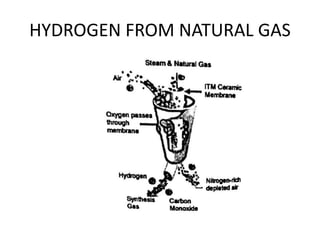
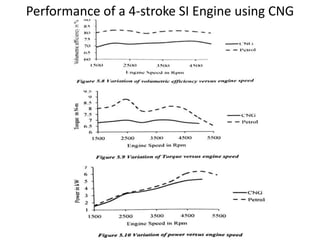
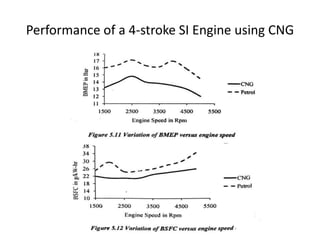
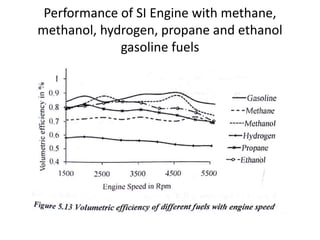
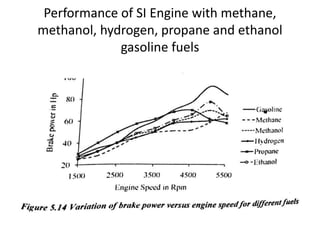
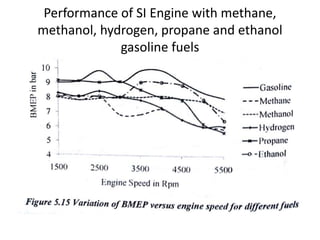
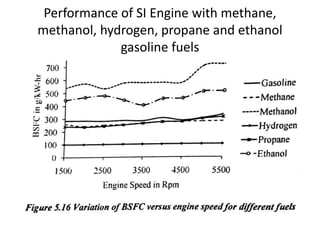
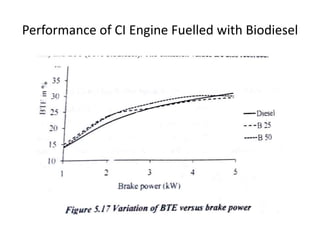
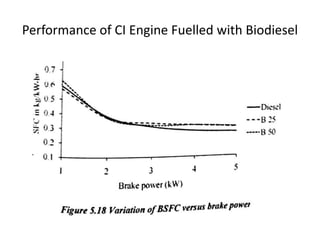
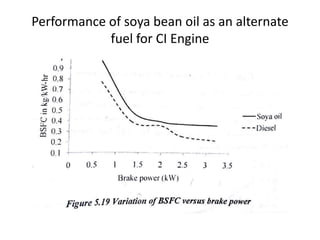
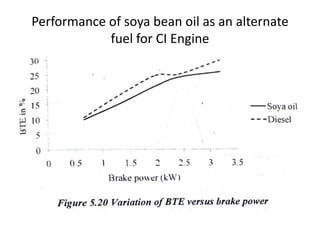
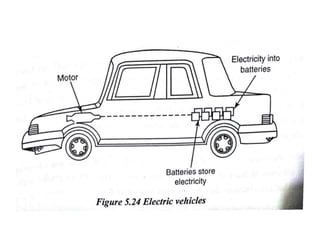
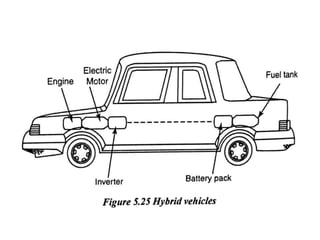
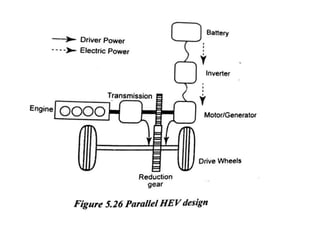
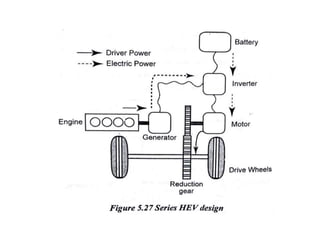
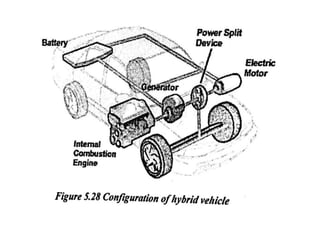
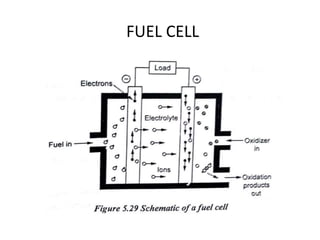
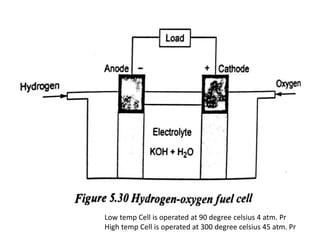
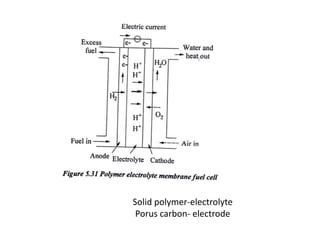
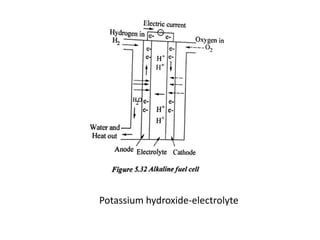
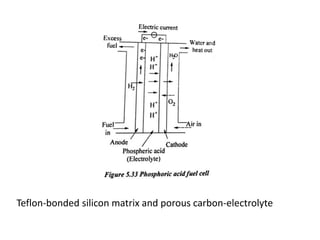
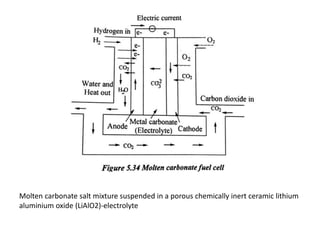
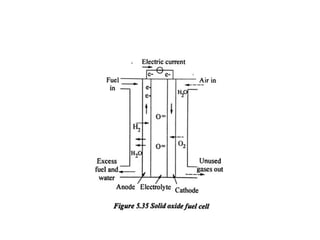
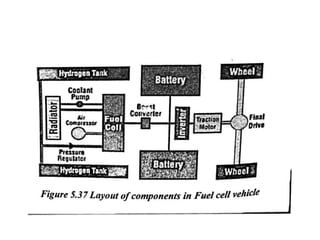
The document discusses various properties of alternative fuels compared to conventional fuels like petrol and diesel. It covers energy density, volatility, octane number, cetane number, heat of vaporization, flame speed, flame temperature, auto-ignition temperature, flash point and flammability limits of different alternative fuels. The production processes of natural gas, biodiesel and ethanol are also outlined. Various engine modifications required for running vehicles on alternative fuels like CNG, biodiesel, bioethanol are described. Performance characteristics of engines running on these alternative fuels are discussed and different types of fuel cells are listed along with brief descriptions.