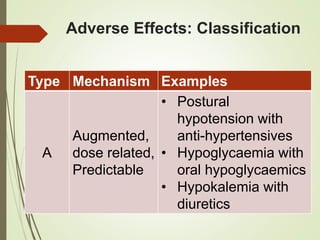
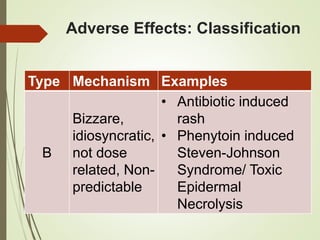
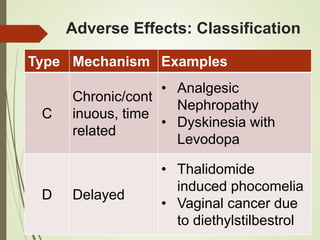
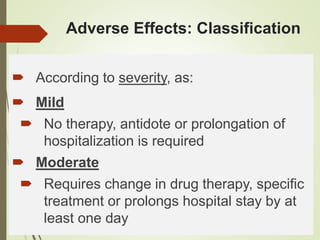
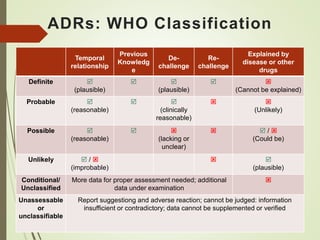
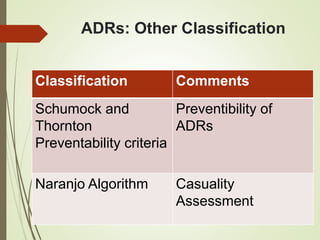
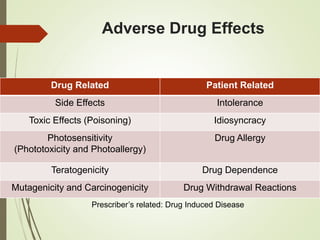
This document discusses adverse drug reactions and their classification. It defines key terms like adverse drug effects, adverse drug events, and adverse drug reactions. It describes different types of adverse reactions based on their mechanism, including type A reactions which are augmented and dose-related, and type B reactions which are idiosyncratic and non-predictable. Adverse reactions are also classified based on their severity as mild, moderate, severe or lethal. The document discusses various classification systems for adverse reactions from WHO and others and covers factors like preventability. It also lists different types of drug related adverse effects patients can experience.