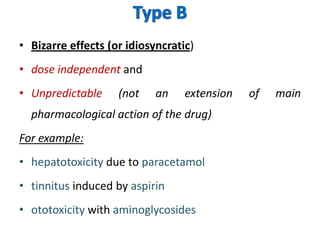
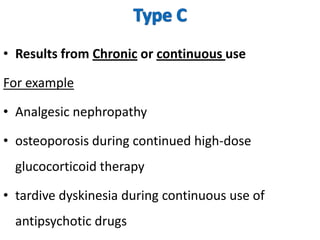
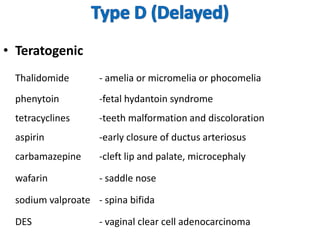
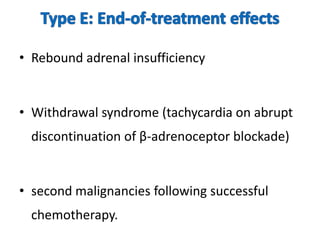
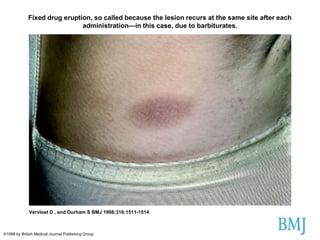
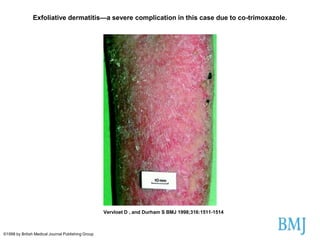
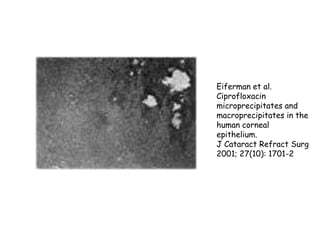
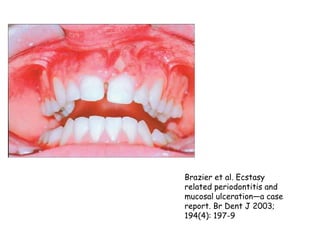
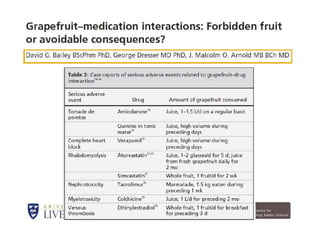
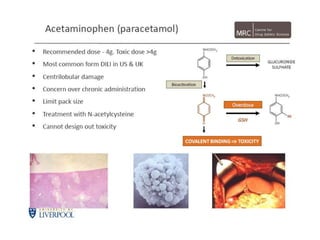
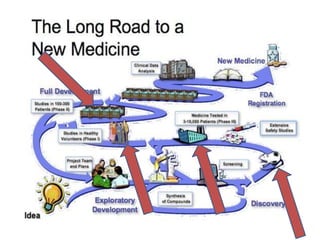
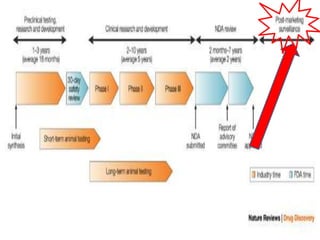
Thalidomide was a sedative that was prescribed to pregnant women in the late 1950s to treat morning sickness. It was later found to cause severe birth defects in over 10,000 babies worldwide, with malformed limbs being the most common defect. Thalidomide was withdrawn from the market in 1961 after the link to birth defects was established. This tragedy highlighted the need for rigorous safety testing of drugs, especially for teratogenic effects, and led to the establishment of regulatory frameworks for drug approval and pharmacovigilance systems like the Yellow Card Scheme to monitor adverse drug reactions.