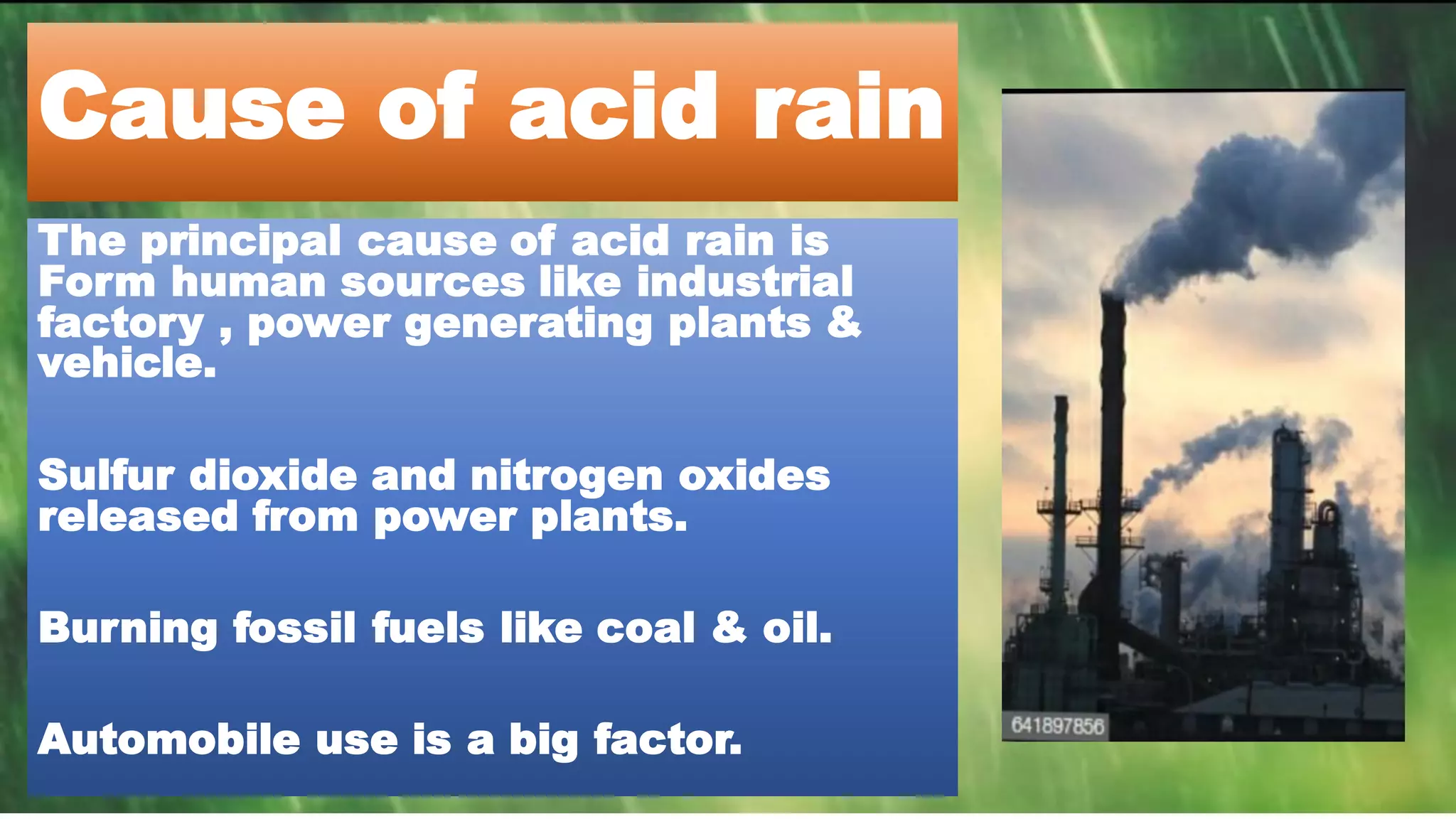
Acid rain occurs when sulfur dioxide and nitrogen oxides react in the atmosphere with water and other chemicals to form acidic compounds like nitric acid and sulfuric acid. The principal cause of acid rain is emissions from industrial factories, power plants, and vehicles that burn fossil fuels like coal and oil. Acid rain damages plants, aquatic ecosystems, infrastructure like buildings and statues, and can impact human health. Suggestions to reduce acid rain include using cleaner energy sources like solar power, reducing vehicle emissions through measures like proper maintenance and using public transportation.
![C.U.Shah Science College
Presented by : Tank Jay P. [1862]
(Biochemistry)](https://image.slidesharecdn.com/acidrainpptbiochem-220917185343-bdb3cf19/75/ACID-RAIN-ppt-biochem-pdf-1-2048.jpg)