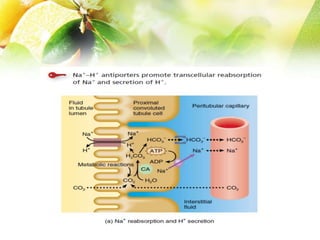
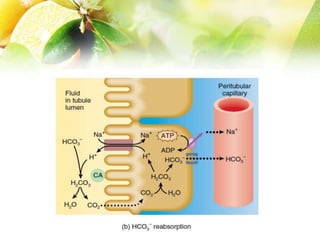
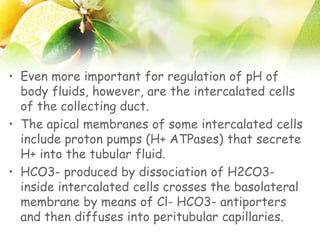
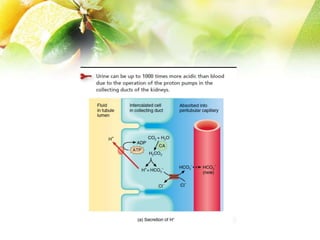
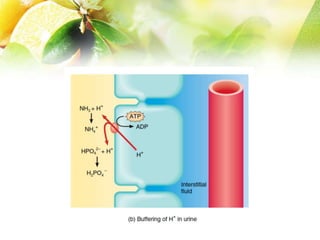
The document discusses the regulation of acid-base balance in the kidneys, highlighting how metabolic processes produce acids that are excreted as hydrogen ions in urine. It explains metabolic acidosis and alkalosis, including their causes and treatments, and emphasizes the importance of urine acidification for preventing renal stones and promoting antibacterial action. Key mechanisms and conditions affecting bicarbonate levels and pH regulation in the body are detailed.