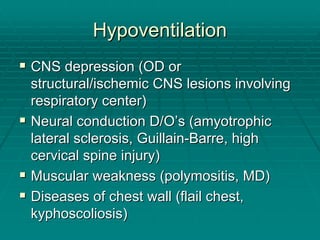
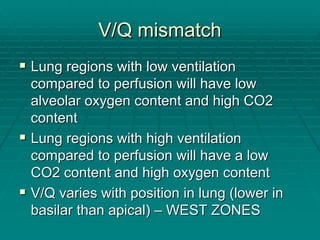
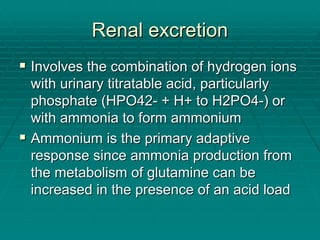
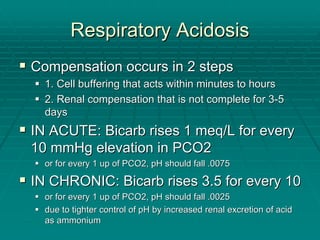
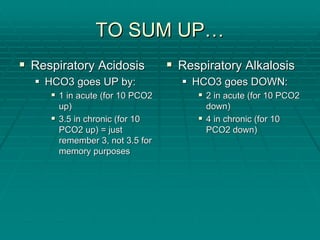
The document provides a comprehensive overview of acid/base balance and arterial blood gas interpretation, detailing important calculations and physiological principles. It explains the significance of the A-a gradient, explains various causes of hypoxemia and acid/base disorders, and discusses compensation mechanisms of respiratory and metabolic acidosis/alkalosis. The document emphasizes a systematic approach for analyzing acid/base problems and calculating relevant parameters like the anion gap.