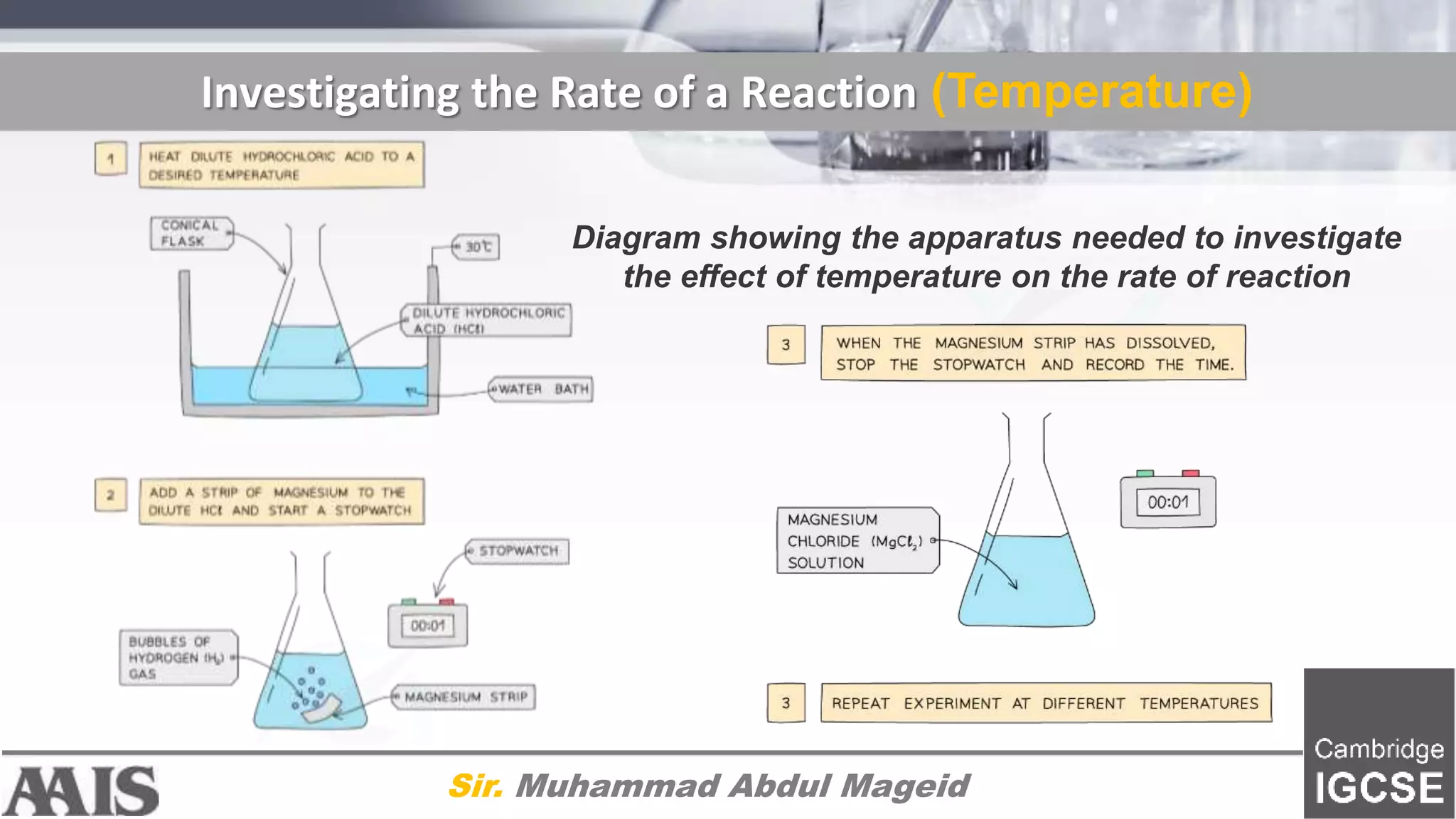
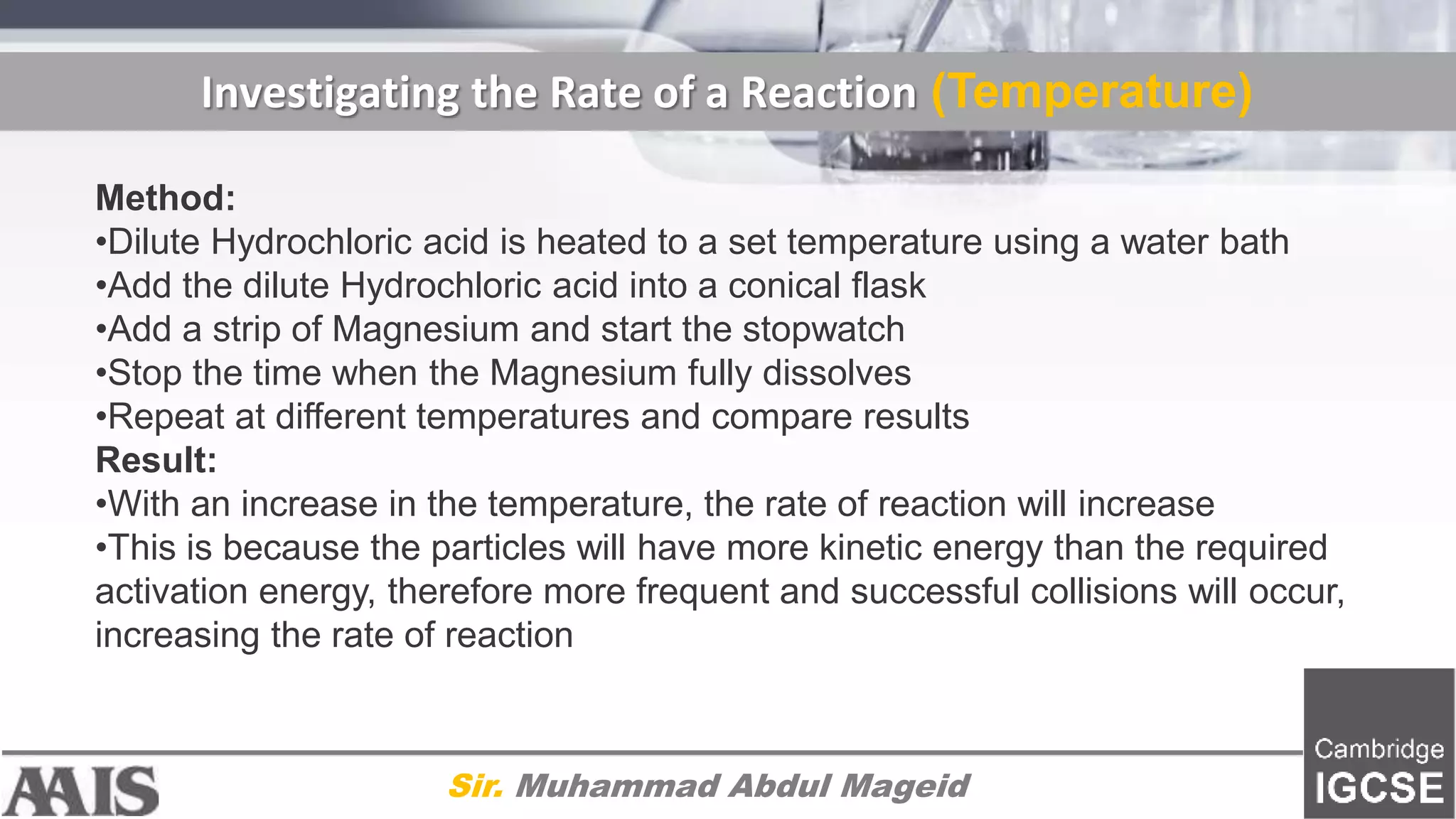
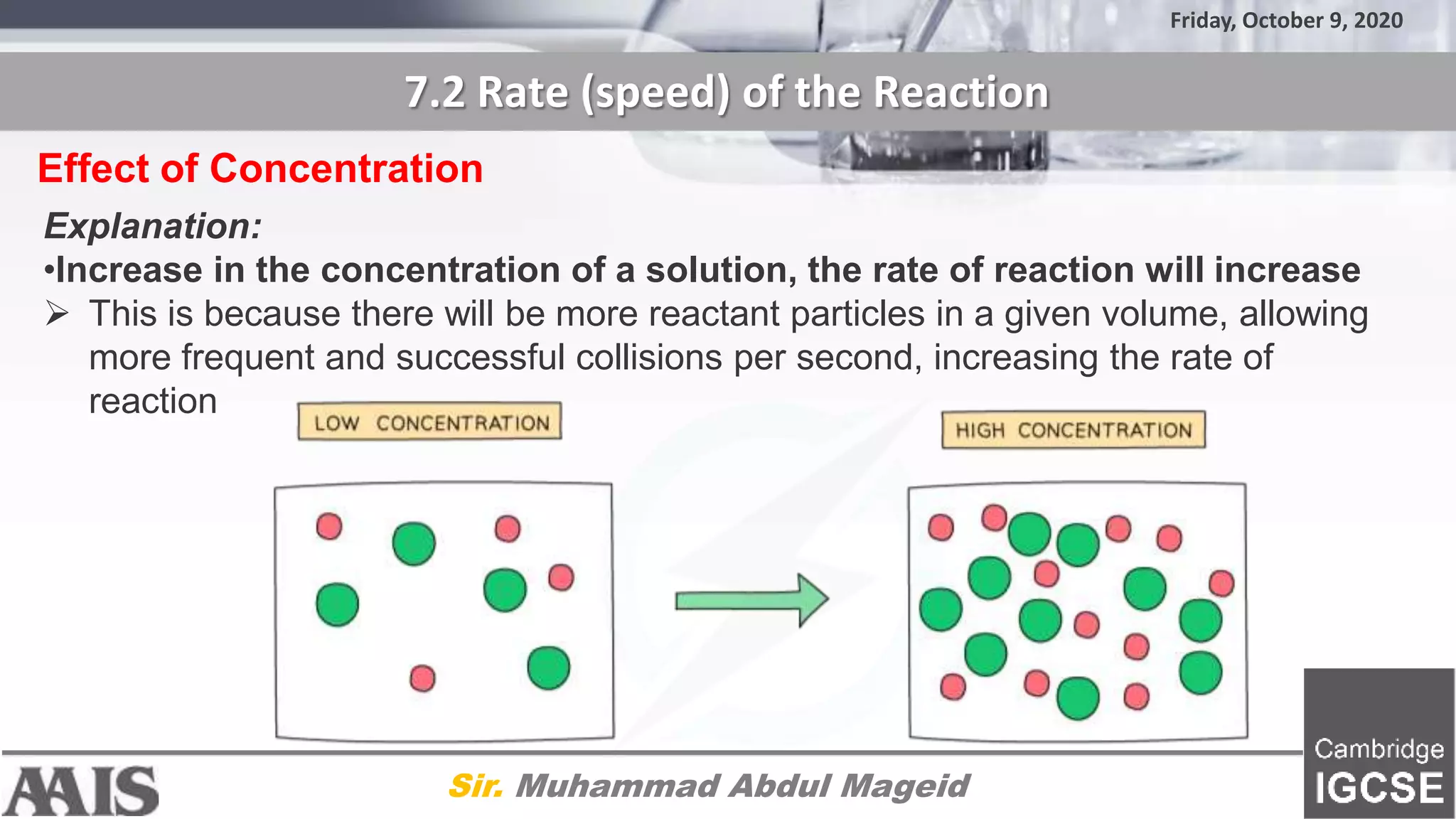
This document discusses factors that affect the rate of chemical reactions, including concentration, temperature, surface area, and use of catalysts. It provides explanations for how increasing concentration, temperature, and surface area increases the rate of reaction by allowing more frequent collisions. It also explains that catalysts increase the rate by lowering the activation energy needed for reactions. The document includes diagrams and methods for investigating how these factors impact the rate of different reactions through experiments.









![Interpreting Data [CONCENTRATION]
Sir. Muhammad Abdul Mageid
Graph showing the effect of
the concentration of a solution
on the rate of reaction
Explanation:
•Compared to a reaction with a reactant
at a low concentration, the graph line for the
same reaction but at a higher concentration
has a steeper gradient at the start and
becomes horizontal sooner
•This shows that with increased
concentration of a solution, the rate of
reaction will increase](https://image.slidesharecdn.com/7-201009163713/75/7-2-chemical-reactions-factors-affecting-rate-of-chemical-reaction-11-2048.jpg)
![Interpreting Data [PARTICLE SIZE]
Sir. Muhammad Abdul Mageid
Graph showing the effect of the surface area of a solid on the rate of reaction
Explanation:
•Compared to a reaction with lumps of reactant,
the graph line for the same reaction but with
powdered reactant has a steeper gradient at the
start and becomes horizontal sooner
•This shows that with increased surface area
of the solid, the rate of reaction will increase](https://image.slidesharecdn.com/7-201009163713/75/7-2-chemical-reactions-factors-affecting-rate-of-chemical-reaction-12-2048.jpg)
![Interpreting Data [CATALYST]
Sir. Muhammad Abdul Mageid
Graph showing the effect of the use of a catalyst on the rate of reaction
Explanation:
•The diagram shows that when a catalyst is
used, the activation energy is reduced as it
creates an alternative pathway requiring lower
activation energy, allowing more successful
and frequent collisions
•This shows that when a catalyst is used, the
rate of reaction will increase](https://image.slidesharecdn.com/7-201009163713/75/7-2-chemical-reactions-factors-affecting-rate-of-chemical-reaction-13-2048.jpg)
![Interpreting Data [TEMPERATURE]
Sir. Muhammad Abdul Mageid
Graph showing the effect of temperature on the rate of reaction
Explanation:
Compared to a reaction at a low temperature, the
graph line for the same reaction but at a higher
temperature has a steeper gradient at the start and
becomes horizontal sooner
This shows that with increased temperature, the
rate of reaction will increase](https://image.slidesharecdn.com/7-201009163713/75/7-2-chemical-reactions-factors-affecting-rate-of-chemical-reaction-14-2048.jpg)