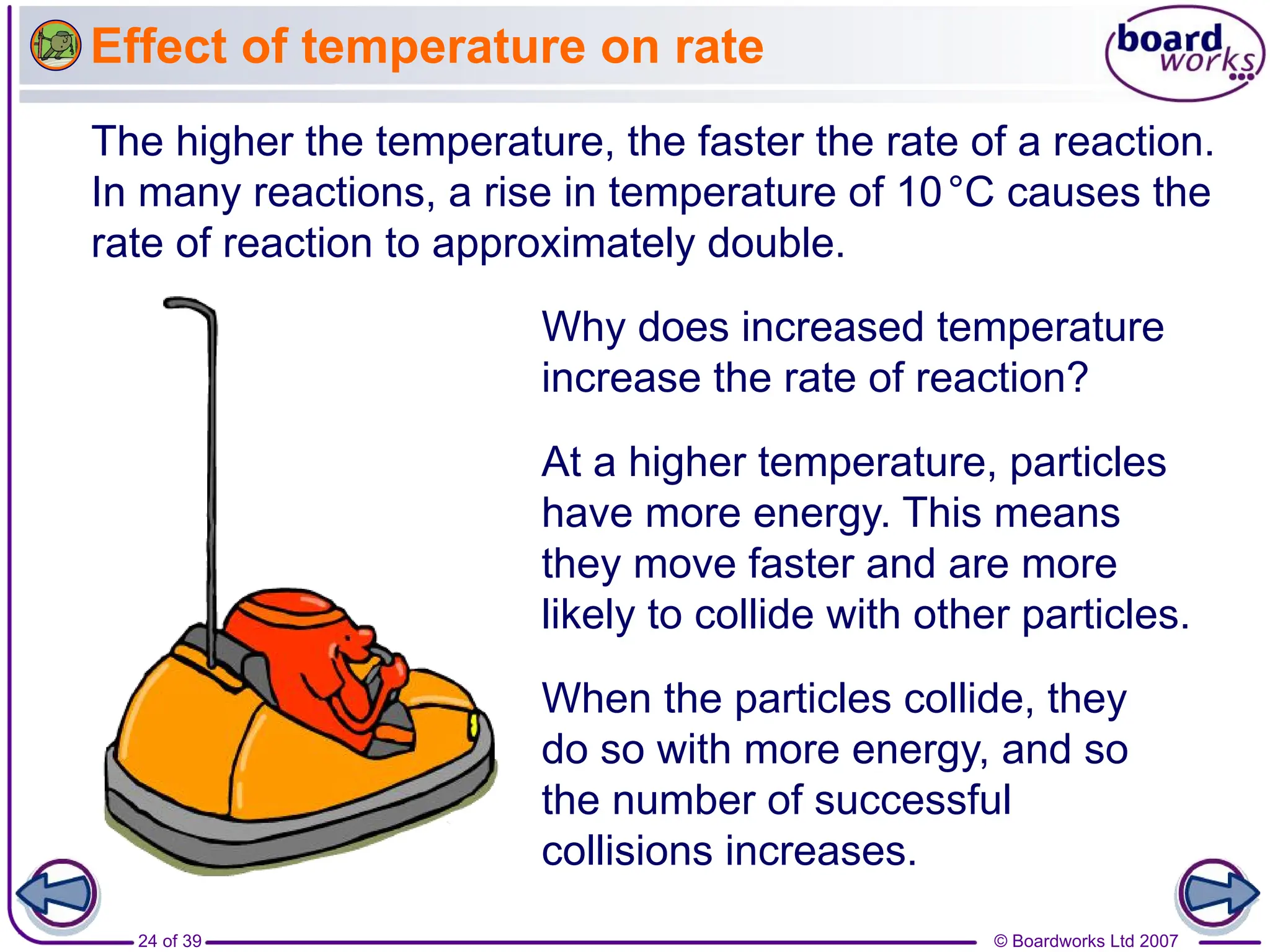
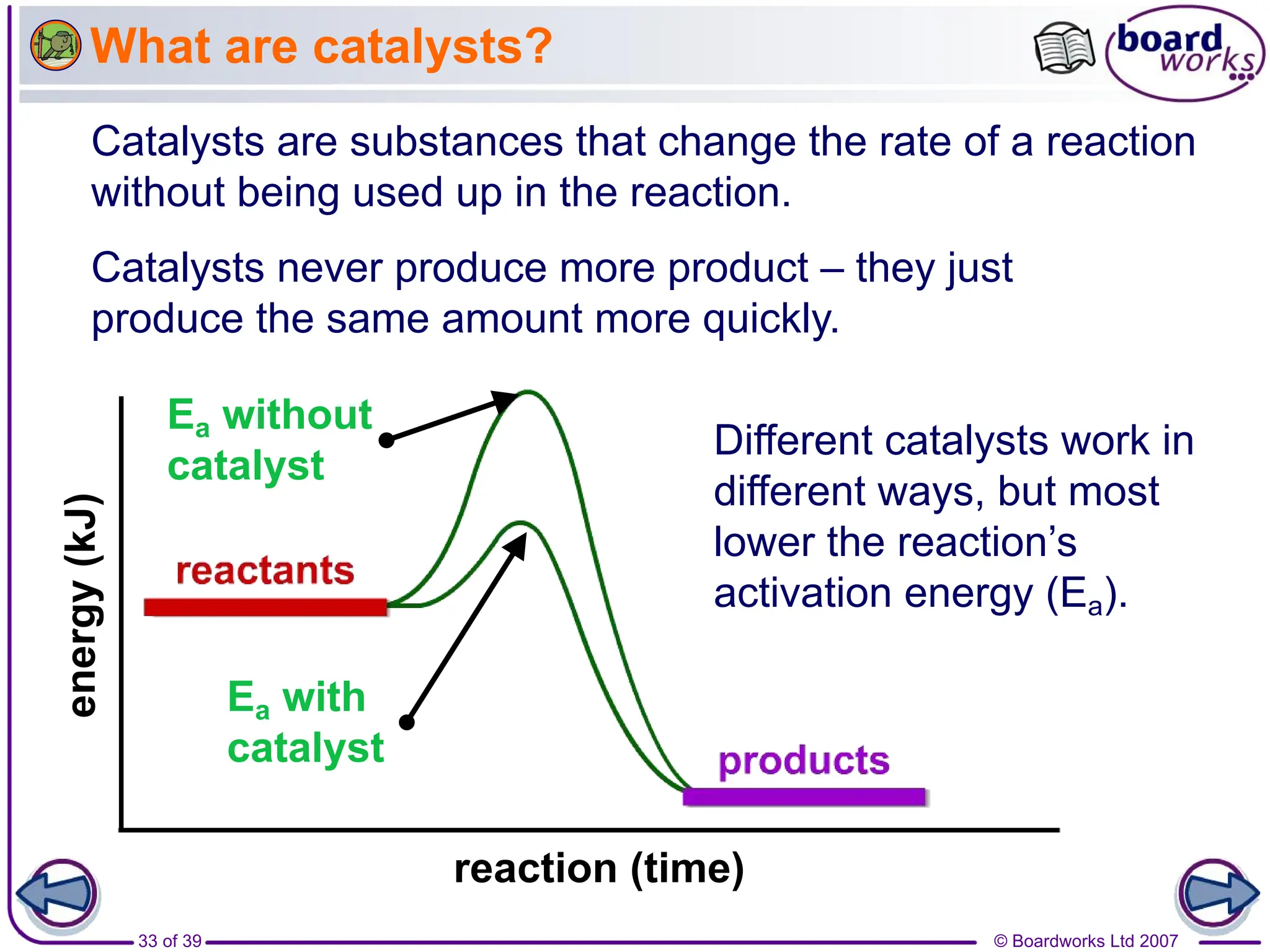
1. The document discusses factors that affect the rate of chemical reactions, including temperature, concentration, pressure, surface area, and catalysts.
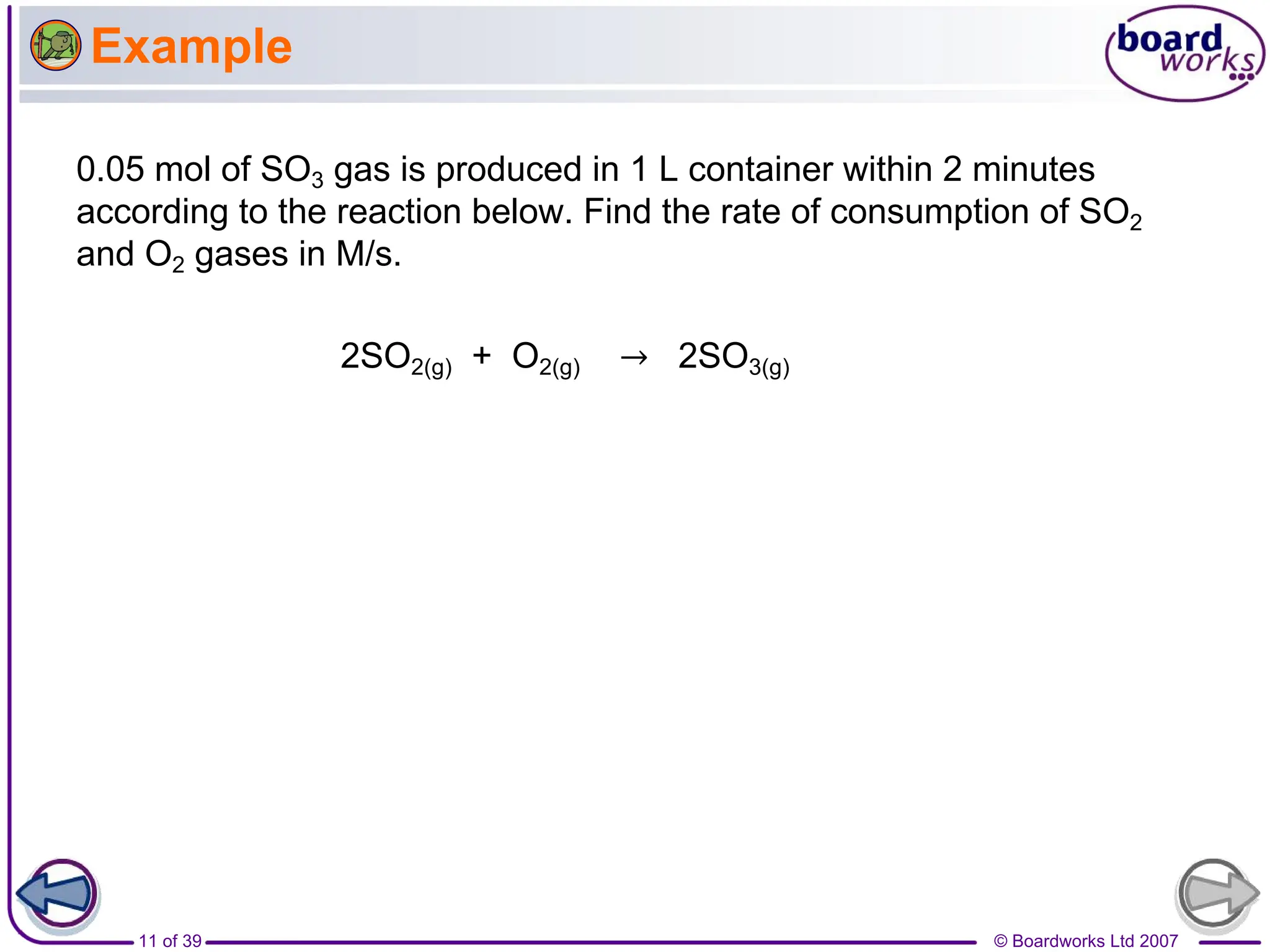
2. It explains that the rate of a reaction is measured by how concentration changes over time, and that rate laws use rate constants and reaction orders.
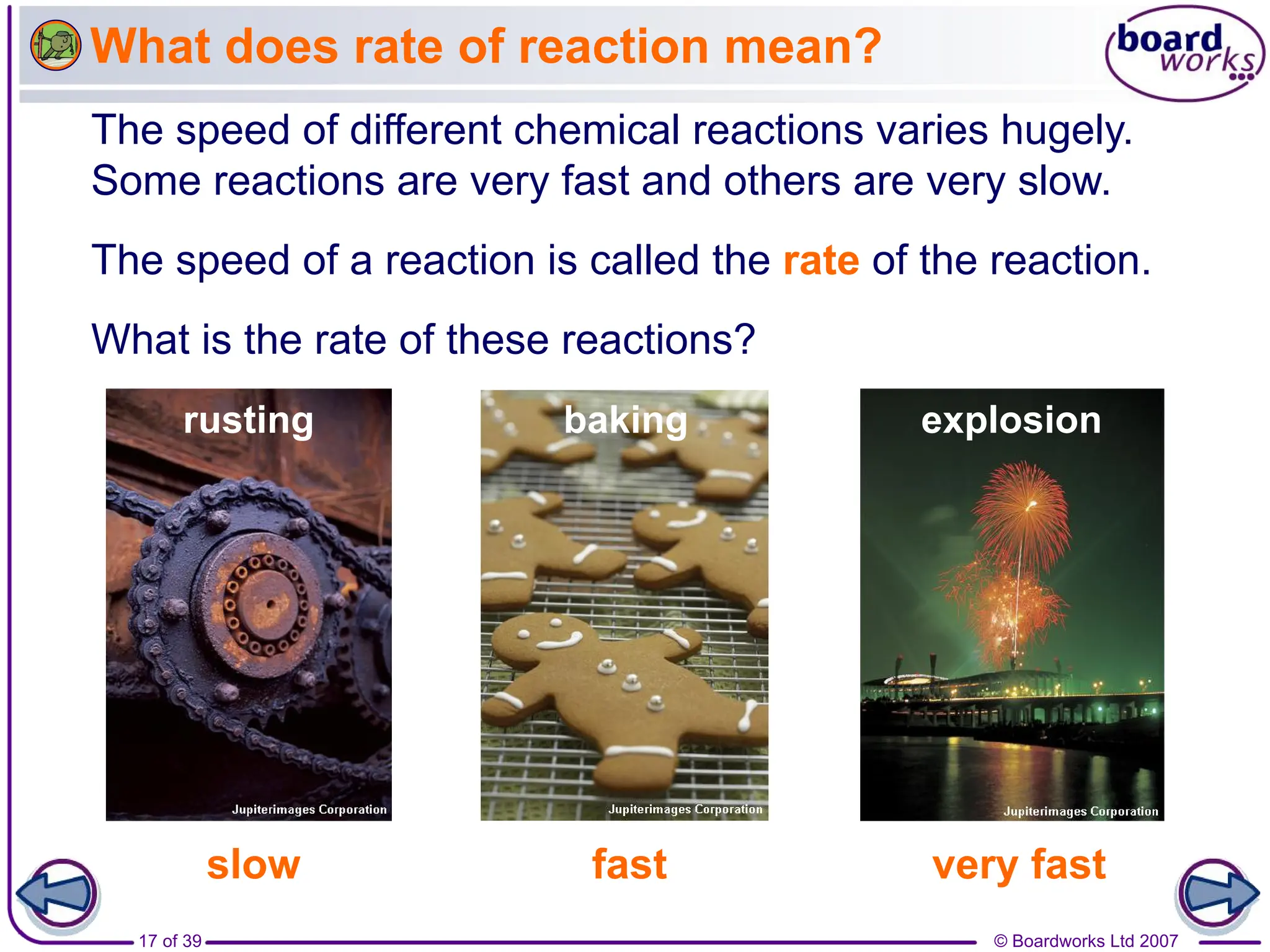
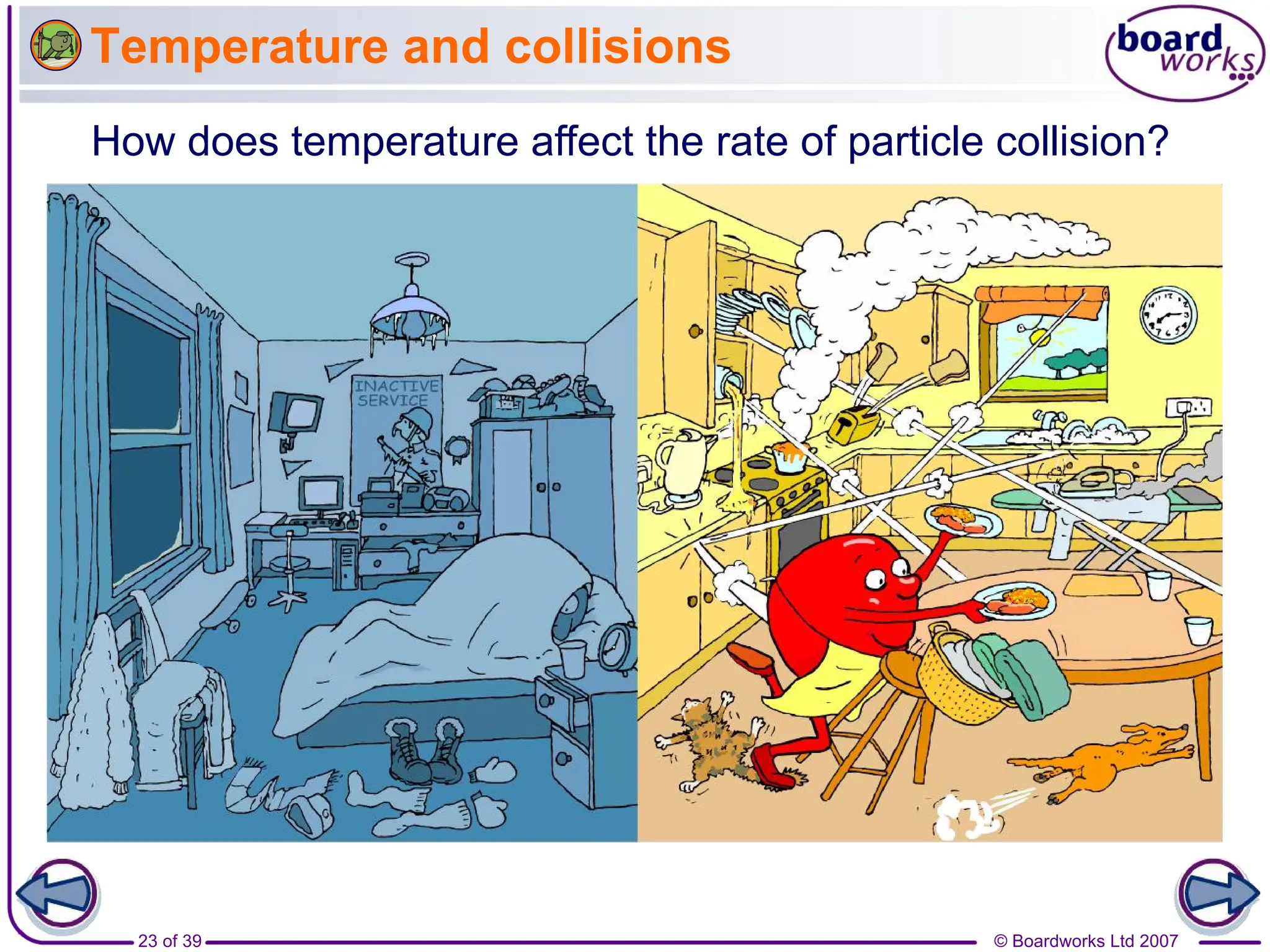
3. Different factors like increased temperature, concentration, or pressure speed up reactions by increasing the frequency of successful collisions between reactant particles.






![© Boardworks Ltd 2007
8 of 39
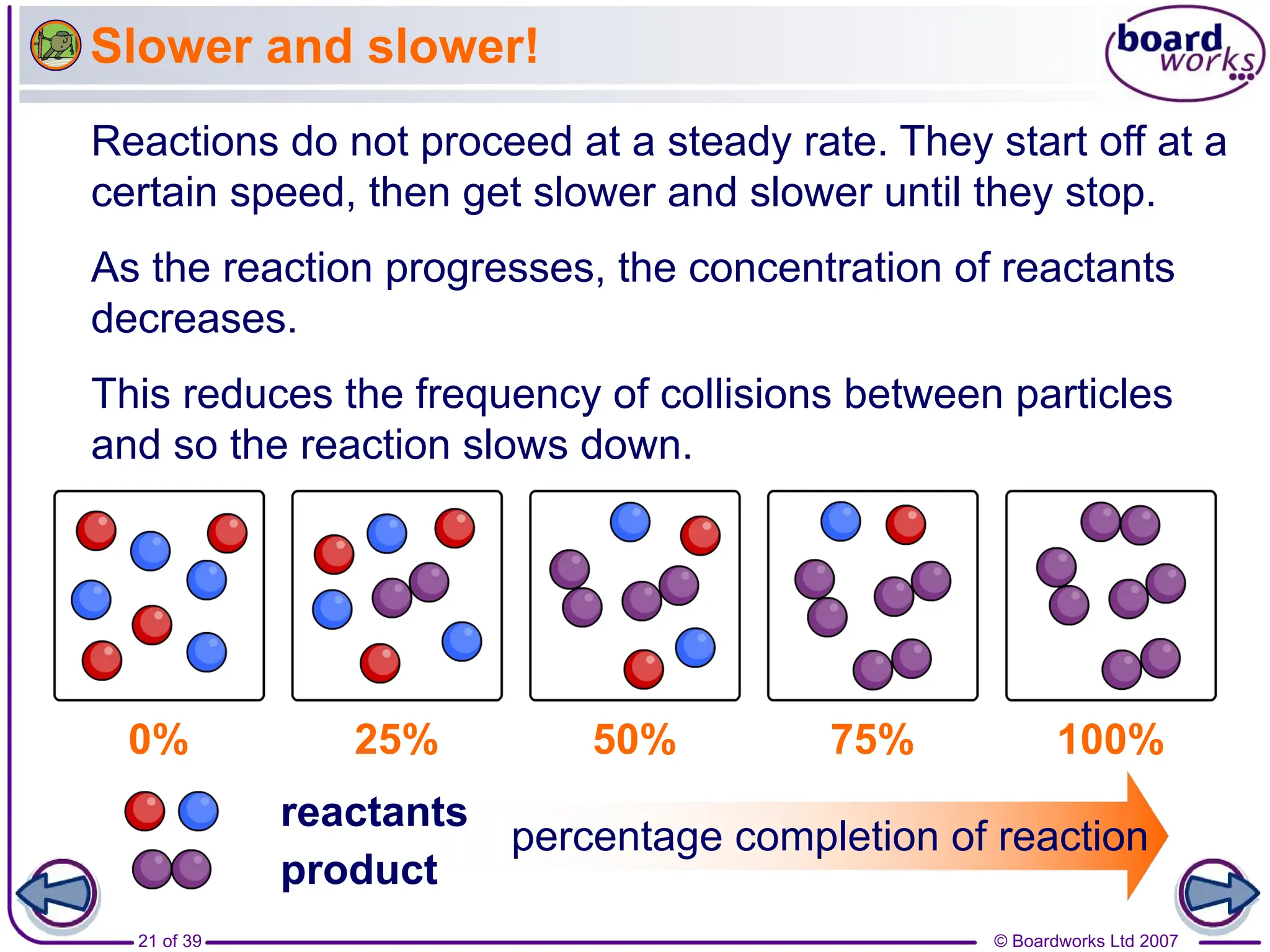
• When the reaction takes places, the amount of reactant
A & B decreases and the amount of product C & D
increases.
Meaning & Measurement of Rate of Reactions
t
[D]
D
D
of
reaction
of
Rate
t
[C]
C
C
of
reaction
of
Rate
t
[B]
-
B
B
of
reaction
of
Rate
t
[A]
-
A
A
of
reaction
of
Rate
](https://image.slidesharecdn.com/rateofreaction-240122005558-318dfd48/75/Rate-of-Reaction-pdf-8-2048.jpg)




![© Boardworks Ltd 2007
14 of 39
Rate Expression and Rate Constant
• For reaction
A + B → product
The rate law is stated in the form of the rate equation as
follows :
V = k [A]x [B]y
Where,
V = rate of reaction (M/s)
k = rate of reaction constant
[A] = concentration of A
[B] = concentration of B
x = order of reaction in A
y = order of reaction in B
x + y = total order](https://image.slidesharecdn.com/rateofreaction-240122005558-318dfd48/75/Rate-of-Reaction-pdf-14-2048.jpg)