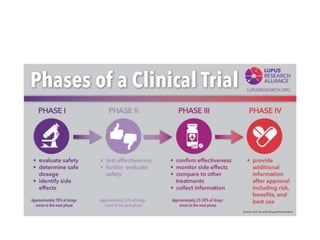
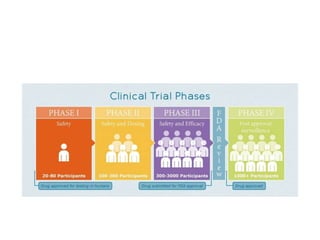
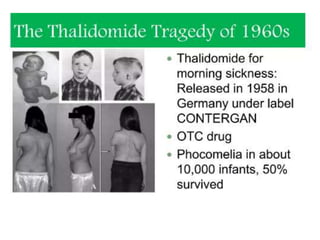
Medical research is vital for the development of new treatments and understanding the functioning of the human body, as well as addressing ethical considerations in healthcare. Physicians must stay updated on research findings to apply them in practice, with a structured clinical trial process required for new drug approvals. Ethical guidelines, such as the Nuremberg Code and the Declaration of Helsinki, mandate informed consent and ensure research is justified and conducted responsibly.
















![• Other, much more detailed, documents have been
produced in recent years on research ethics in general
• e.g., Council for International Organizations of Medical
Sciences, International Ethical Guidelines for
Biomedical Research Involving Human Subjects,
1993, revised in 2002)
• Specific topics in research ethics (e.g., Nuffield Council
on Bioethics [UK], The Ethics of Research Related to
Healthcare in Developing Countries, 2002).](https://image.slidesharecdn.com/9-230220072922-31b630a7/85/9-Medical-Research-pptx-20-320.jpg)







