The document describes an efficient method for direct arylsulfonamidation of aryl iodides and bromides using arylsulfonylazides catalyzed by copper nanoparticles. Aryl halides and arylsulfonylazides are reacted in DMF with copper nanoparticles, K2CO3, and a ligand at 110°C. The reaction produces various N-arylsulfonamides in moderate to good yields. The copper nanoparticles are prepared according to a literature procedure.

![1 | P a g e
Nanocopper-mediated Direct Arylsulfonamidation of Aryl
Halides with Arylsulfonylazides
I. Yavari*
and Y. Solgi, M. Ghazanfarpour-Darjani, S. Ahmadian
Department of Chemistry, Tarbiat Modares University, PO Box 14115-175, Tehran, Iran
An efficient protocol for direct arylsulfonamidation of aryl iodides and aryl bromides using
arylsulfonylazides catalyzed by copper nanoparticles is described.
Keywords: Arylsulfonyl azide, Aryliodide, Arylchloride, Cu NPs
*Corresponding author. E-mail: yavarisa@modares.ac.ir
INTRODUCTION
The copper-mediated C(aryl)-N bond formation is an important transformation in organic
synthesis and has been developed to include a wide range of substrates [1-3]. The foundation of
modern copper-mediated chemistry lies in the pioneering work of Ullmann and Goldberg [4].
Buchwald and co-workers extensively studied these reactions and developed an experimentally
simple and inexpensive catalytic system for these transformations [5-7].
N-Arylsulfonamide moiety is an important structural motif in many pharmaceutical
compounds [8]. Transition-metal-catalyzed N-arylation of sulfonamides offers a straightforward
method for the preparation of N-arylsulfonamides. Recently, the Cu-catalyzed coupling of aryl-
Manuscript
Click here to download Manuscript: Manuscript JICS.docx.docx Click here to view linked References
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
61
62
63
64
65](https://image.slidesharecdn.com/c1b776fd-1b6f-4c30-8ad1-f995f681ca5a-170118143932/85/5-2-320.jpg)
![2 | P a g e
boronic acids, as well as, the Pd-catalyzed coupling of aryl bromides and aryl chlorides with
sulfonamides has been reported [9-13]. Most of the methods developed for synthesis of N-
arylsulfonamide derivatives are often troublesome and require strong bases, and expensive Pd
catalysts [14-17].
EXPERIMENTAL
All chemicals were obtained commercially and used without further purification. M.p.:
Electrothermal-9100 apparatus. IR Spectra: Shimadzu-IR-460 spectrometer; in cm−1
. 1
H- and
13
C-NMR Spectra: Bruker DRX-500 Avance instrumentat 500.1 and 125.7 MHz, resp.; δ in ppm,
J in Hz. MS: Finnigan-MAT-8430EI-MS mass spectrometer; at 70 eV; in m/z (rel. %). Elemental
analyses: Vario EL III CHNOS elemental analyzer. Scanning electron microscopy (SEM) images
were obtained using a Stereo Scan XL30 Philips.
General procedure
To a mixture of aryl halide 1 (1 mmol), sulfonylazide 2 (1.2 mmol), and CuI (0.1 mmol), ligand
(0.2 mmol) and K2CO3 (2 mmol) in DMF (4 mL) was added nano-Cu (53 nm), (0.5 mmol). The
mixture was stirred at 110 °C. After completion of the reaction [(24 h; TLC (AcOEt/hexane 1:5)
monitoring)], the mixture was diluted with CH2Cl2 (7 mL) and aqueous NH4Cl solution (5 mL),
stirred for 30 min and the layers separated. The aqueous layer was extracted with CH2Cl2 (5 mL
× 3) and the combined organic fractions dried (Na2SO4) and concentrated under reduced
pressure. Pure products were obtained by flash column chromatography [(silica gel (230–400
mesh; Merck), hexane/AcOEt 5:1)]. The copper nanoparticles were prepared according to
literature [19]. Compounds 3a [11], 3b [11], 3d [10], 3l [10], and 3n [9] are known.
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
61
62
63
64
65](https://image.slidesharecdn.com/c1b776fd-1b6f-4c30-8ad1-f995f681ca5a-170118143932/85/5-3-320.jpg)



![6 | P a g e
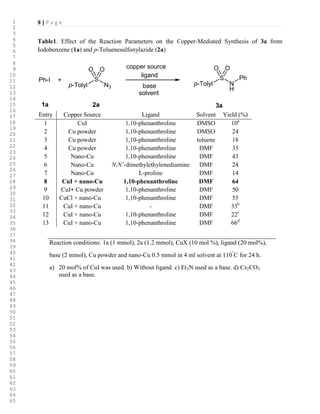
RESULTS AND DISCUSSION
Copper powder is reported [18] to have been effective catalyst for the decomposition of
arylsulfonylazides. We attempted to use this reaction conditions for coupling of the sulfonamide
produced by decomposition of sulfonylazides and aryl halides and design a novel an efficient
one-pot synthesis of N-arylsulfonamides. We initially selected iodobenzene (1a), (1 equiv) and
p-toluenesulfonylazide (2a), (1.2 equiv) as the model substrate for optimization of the reaction
conditions (Scheme 1). Then, the catalysis conditions, including copper source, ligand, base and
solvent were optimized. Our first test was performed in DMSO using a catalytic amount of CuI
(0.2 equiv), K2CO3 (2 equiv) as the base and 1,10-phenanthroline (0.2 equiv) as the ligand at
110°
C. Under these conditions, only small amounts (10%) of product (3a) were detected.
Under these reaction conditions, a set of catalysts such as CuCl, Cu2O and Cu(OAc)2
were screened, without success. We were pleased to discover that 0.5 equiv of copper powder
could afford the desired product in 24% yield. The same reaction in toluene afforded 3a in 18%
yield. Fair to good yields of 3a were obtained with DMF as the solvent. In this solvent a brief
study of several ligands, bases and copper sources were carried out. A significant improvement
was achieved by using a combination of CuI, nano-Cu powder [19] (53 nm, Figure 1) and 1,10-
phenanthroline. The results are summarized in Table 1.
>Table 1<
Thus, the optimized reaction condition was started up by using 1equiv of iodobenzene,
1.2 equiv of p-toluenesulfonylazide, 10 mol-% CuI, 0.5 equiv. nano-Cu powder, 20 mol% 1,10-
phenanthroline and 2 equiv of K2CO3 in DMF at 110°
C (Scheme 2).
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
61
62
63
64
65](https://image.slidesharecdn.com/c1b776fd-1b6f-4c30-8ad1-f995f681ca5a-170118143932/85/5-7-320.jpg)
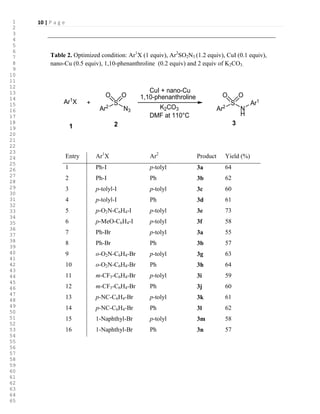
![7 | P a g e
As shown in Table 2, using the optimized conditions described above, various N-
arylsulfonamides were synthesized from aryl iodides, aryl bromides and arylsulfonylazides. A
wide variety of functional groups, including nitro, cyano and trifluoromethyl, are tolerated by
these conditions. Arylsulfonylazides worked well as substrates, and the corresponding products
were obtained in good yield.
>Table 2<
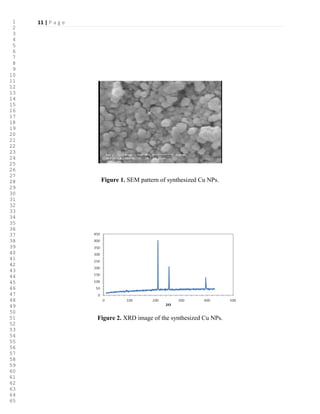
Copper nanoparticles used in this study, have been prepared according to literature [19].
Figure 1 shows XRD pattern of the Cu NPs. All of the diffraction peaks are in agreement with
the JCPDS file of Cu [19]. The morphology and grain size of the Cu NPs were investigated by
SEM (Figure 2). They have spherical morphology including a narrow distribution of sizes, from
32 nm to 68 nm.
>Figure 1<
>Figure 2<
CONCLUSIONS
In conclusion, we report an efficient synthetic procedure for the direct C-N bond formation
catalyzed by simple copper sources to form the corresponding N-arylsulfonamides by using aryl
halides and sulfonylazides. This method has advantages such as offering a simple and
inexpensive catalyst and simple workup.
REFERENCES
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
61
62
63
64
65](https://image.slidesharecdn.com/c1b776fd-1b6f-4c30-8ad1-f995f681ca5a-170118143932/85/5-8-320.jpg)
![8 | P a g e
[1] G. Evano, N. Blanchard, M. Toumi, Chem. Rev. 108 (2008) 3054.
[2] F. Monnier, M. Taillefer, Angew. Chem. Int. Ed. 48 (2009) 2.
[3] S. V. Ley, A. W. Thomas, Angew. Chem. Int. Ed. 42 (2003) 5400.
[4] F. Ullmann, Ber. Dtsch. Chem. Ges. 36 (1903) 2382.
[5] A. Kiyomori, J. Marcoux, S. L. Buchwald, Tetrahedron Lett. 40 (1999) 2657.
[6] A. Shafir, S. L. Buchwald, J. Am. Chem. Soc. 128 (2006) 8742.
[7] E. R. Strieter, B. Bhayana, S. L. Buchwald, J. Am. Chem. Soc. 131 (2009) 78.
[8] A. Scozzafava, T. Owa, A. Mastrolorenzo, C. T. Supuran, Curr. Med. Chem. 10 (2003) 925.
[9] G. Burton, P. Cao, G. Li, R. Rivero, Org. Lett. 5 (2003) 4373
[10] P. Y. S. Lam, G. Vincent, C. G. Clark, S. Deudon, P. K. Jadhav, Tetrahedron Lett. 42
(2001) 3415.
[11] N. Liu, B. Tang, Y. Chen, L. He, Eur. J. Org. Chem. (2009) 2059.
[12] G. H. Imanzadeh, A. Zare, A. Khalafi-Nezhad, A. Hasaninejad, A. R. Moosavi-Zare,
A.
Parhami, J. Iran. Chem. Soc. 4 (2007) 467.
[13] A. R. Massah, M. Dabagh, S. Shahidi, H. Javaherian-Naghash, A. R. Momeni, H. Aliyan, J.
Iran. Chem. Soc. 6 (2009) 405.
[14] H. He, Y. J. Wu, Tetrahedron Lett. 44 (2003) 3385.
[15] D. Steinhuebel, M. Plaucki, D. Askin, U. Dolling, Tetrahedron Lett. 45 (2004) 3305.
[16] W. Deng, L. Liu, C. Zhang, M. Liu, Q. X. Guo, Tetrahedron Lett. 46 (2005) 7295.
[17] L. Alcaraz, C. Bennion, J. Morris, P. Meghani, S. M. Thom, Org. Lett. 6 (2004) 2705.
[18] H. Kwart, A. A. Kahn, J. Am. Chem. Soc. 89 (1967) 1950.
[19] S. Wu, D. J. Chen, Colloid. Interf. Sci. 273 (2004) 165.
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
61
62
63
64
65](https://image.slidesharecdn.com/c1b776fd-1b6f-4c30-8ad1-f995f681ca5a-170118143932/85/5-9-320.jpg)