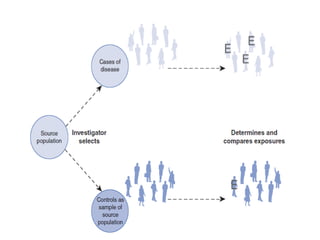
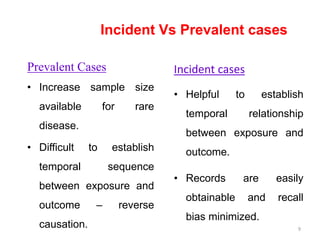
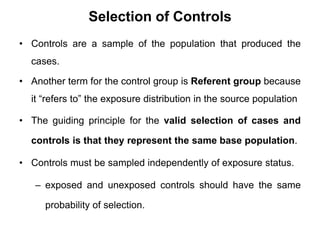
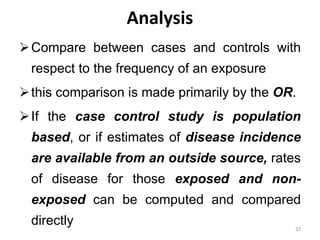
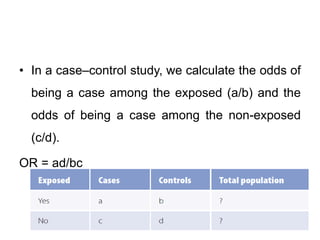
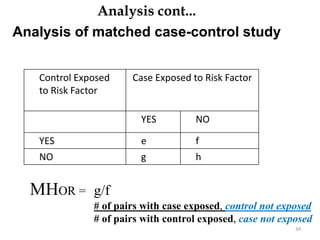
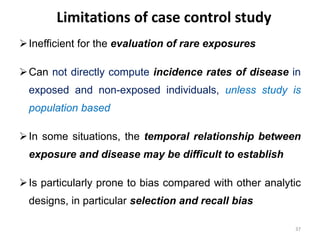
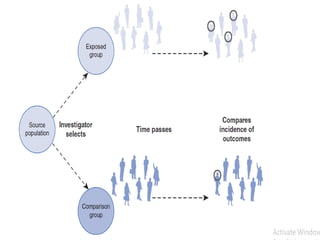
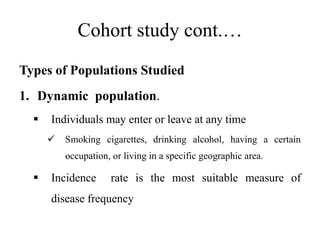
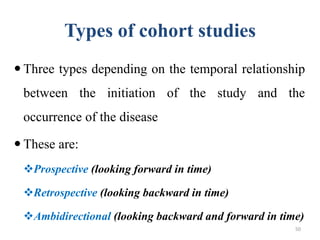
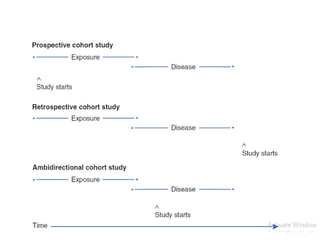
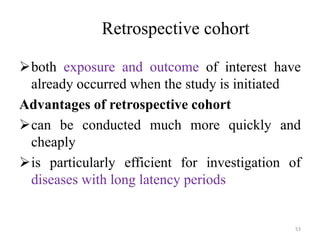
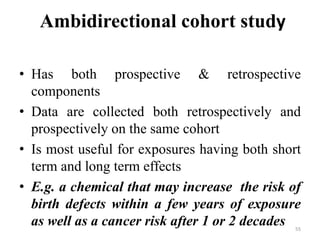
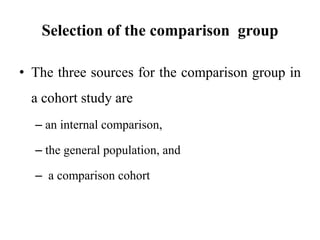
This document provides an overview of case-control studies. It defines a case-control study as one where subjects are selected based on whether they have or do not have a particular disease, and then compared with respect to exposure history. It discusses when case-control studies are desirable, how to select cases and controls, sources of cases and controls, ascertainment of disease and exposure status, and analysis. The key aspects covered include definition, study design, selection of cases and controls, and methodology.