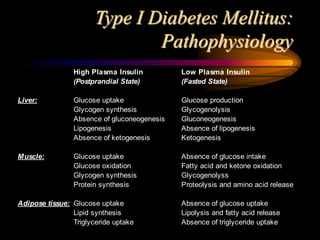
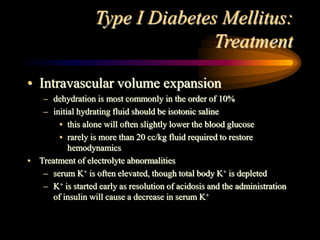
The document discusses diabetic ketoacidosis, providing an overview of its pathophysiology, classification, diagnosis, and treatment. Diabetic ketoacidosis results from a lack of insulin and leads to hyperglycemia, ketonemia, and metabolic acidosis if not treated. Treatment involves fluid resuscitation, electrolyte replacement, insulin therapy to reduce blood glucose levels and resolve acidosis, and careful monitoring to prevent complications such as cerebral edema.