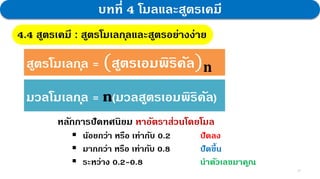
1. Joseph Proust, a French scientist, discovered the Law of Constant Proportions through experiments on chemical reactions forming compounds. He found that the mass ratios of elements in a compound are always fixed, regardless of preparation method.
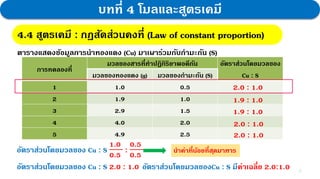
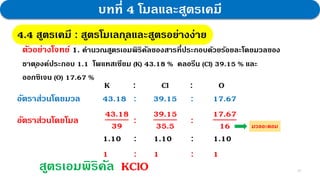
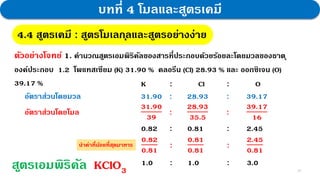
2. Experiments reacting copper and sulfur in different ratios showed that the average mass ratio of Cu to S was 2:1. Calculations determining the molar ratios also gave a ratio of 1:1, confirming the law.
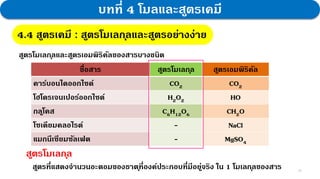
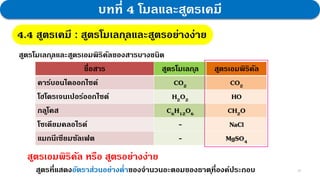
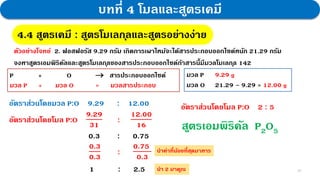
3. Further experiments and calculations on copper(II) oxide supported that its composition follows the law, with the molar ratio of Cu to O always being 1:1.